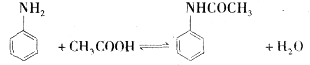
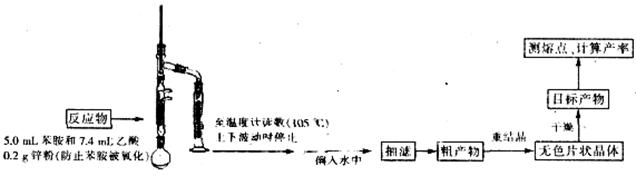
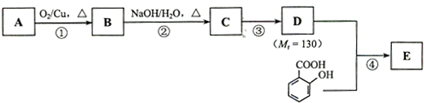
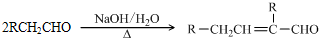��Ŀ����
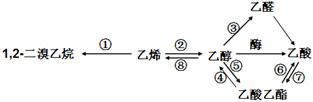
i��֪A����̬������ȫȼ��ʱ������CO2��H2O �����ʵ���֮��Ϊ1��1��A����Է�������С��30������ͼ�仯�У�FΪ�߷��ӻ����C�к���-CHO��E��ˮ������ζ����Ӧ����δд����
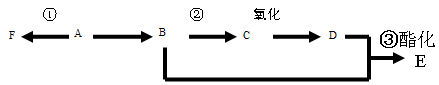
�� B���������������� E ���ʵ�����
�� ��Ӧ������Ϊ
�� д�����з�Ӧ�Ļ�ѧ����ʽ��ע����Ӧ������
�� ��
�� һ�������Ҵ�����������������ȼ�գ��õ�CO��CO2��ˮ��������Ϊ27.6g,������ˮ������Ϊ10.8g,��CO������Ϊ g.
���л���A����ͨ����ͬ��ѧ��Ӧ�ֱ��Ƶ�B��C��D�������ʣ��ṹ��ʽ����ͼ��ʾ��
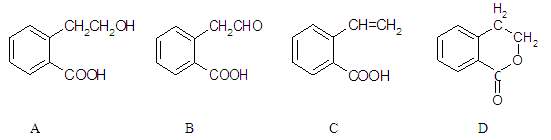
��1��B�еĺ��������������� ��
��2�� A��C�ķ�Ӧ������ ��A��D�л�Ϊͬ���칹����� ��
��3����A����C�Ļ�ѧ����ʽ�� ��
��4��C��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ�� ��

�� B���������������� E ���ʵ�����
�� ��Ӧ������Ϊ
�� д�����з�Ӧ�Ļ�ѧ����ʽ��ע����Ӧ������
�� ��
�� һ�������Ҵ�����������������ȼ�գ��õ�CO��CO2��ˮ��������Ϊ27.6g,������ˮ������Ϊ10.8g,��CO������Ϊ g.
���л���A����ͨ����ͬ��ѧ��Ӧ�ֱ��Ƶ�B��C��D�������ʣ��ṹ��ʽ����ͼ��ʾ��

��1��B�еĺ��������������� ��
��2�� A��C�ķ�Ӧ������ ��A��D�л�Ϊͬ���칹����� ��
��3����A����C�Ļ�ѧ����ʽ�� ��
��4��C��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ�� ��
�����ǻ��������������ƼӾ۷�Ӧ����3���� 2CH3CH2OH + O2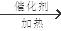 2CH3CHO + 2H2O
2CH3CHO + 2H2O
�� CH3CH2OH + CH3COOH CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O
�� 1.4g
����1��ȩ�����Ȼ�����2����ȥ��Ӧ��C��D��
��3��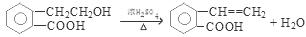
��4��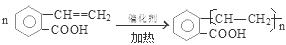
 2CH3CHO + 2H2O
2CH3CHO + 2H2O �� CH3CH2OH + CH3COOH
 CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O �� 1.4g
����1��ȩ�����Ȼ�����2����ȥ��Ӧ��C��D��
��3��
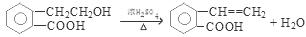
��4��

�������������̬��A��ȫȼ��ʱ������CO2��H2O �����ʵ���֮��Ϊ1��1��A����Է�������С��30��A������C��H=1:2������A��C2H4,���������֮���ת����ϵ��֪��F�Ǿ���ϩ��B���Ҵ�CH3CH2OH��C����ȩCH3CHO��D������CH3COOH��E����������CH3COOHCH2 CH3. �� B�������������������ǻ���E ���ʵ������������������Ǣڷ�Ӧ�Ļ�ѧ����ʽ�ǣ�2CH3CH2OH + O2
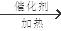 2CH3CHO + 2H2O���۷�Ӧ�Ļ�ѧ����ʽ�ǣ�CH3CH2OH + CH3COOH
2CH3CHO + 2H2O���۷�Ӧ�Ļ�ѧ����ʽ�ǣ�CH3CH2OH + CH3COOH CH3COOCH2CH3 + H2O����n(H2O) =10.8g��18g/mol=0.6mol������ÿ1mol���Ҵ�ȼ�ղ���3mol��ˮ�������Ҵ������ʵ�����0.2mol. CO��CO2��ˮ��������Ϊ27.6g,����ˮ������Ϊ10.8g,��CO��CO2��������16.8g.����CO��CO2�����ʵ����ֱ���x��y,�����C�غ�ɵ�x+y=0.4.�ṹ�����غ�ɵ�28x+44y=16.8.���x=0.05mol��y=0.35mol������CO��������0.05mol��28g/mol=1.4g������1������B�Ľṹ��ʽ��֪��B�еĺ���������������ȩ�����Ȼ�����2��A��C�ķ�Ӧ��������ȥ��Ӧ��ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ�Ļ������A��D�л�Ϊͬ���칹�����C��D����3����A����C�Ļ�ѧ����ʽ��
CH3COOCH2CH3 + H2O����n(H2O) =10.8g��18g/mol=0.6mol������ÿ1mol���Ҵ�ȼ�ղ���3mol��ˮ�������Ҵ������ʵ�����0.2mol. CO��CO2��ˮ��������Ϊ27.6g,����ˮ������Ϊ10.8g,��CO��CO2��������16.8g.����CO��CO2�����ʵ����ֱ���x��y,�����C�غ�ɵ�x+y=0.4.�ṹ�����غ�ɵ�28x+44y=16.8.���x=0.05mol��y=0.35mol������CO��������0.05mol��28g/mol=1.4g������1������B�Ľṹ��ʽ��֪��B�еĺ���������������ȩ�����Ȼ�����2��A��C�ķ�Ӧ��������ȥ��Ӧ��ͬ���칹���Ƿ���ʽ��ͬ���ṹ��ͬ�Ļ������A��D�л�Ϊͬ���칹�����C��D����3����A����C�Ļ�ѧ����ʽ��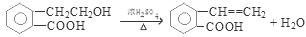 ��4��C��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��
��4��C��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ�� ��
��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
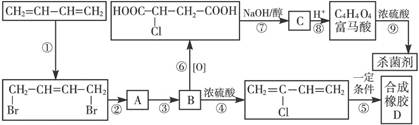
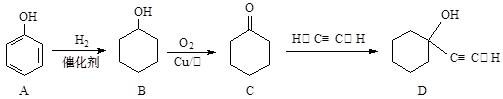
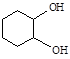 ����Ӧ���������� ��ѡ����ţ���
����Ӧ���������� ��ѡ����ţ���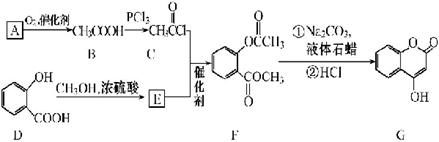
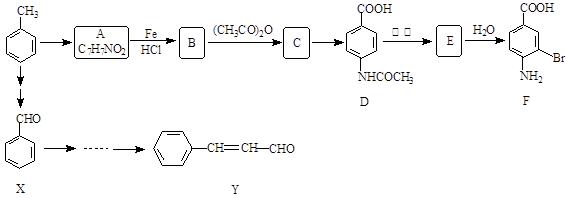
 ��
��