��Ŀ����
��10�֣�ijͬѧ�������е�Na2O2��CO2��Ӧ��֪ʶ����Ǩ�ƣ���ΪNa2O2Ҳ�ɺ�SO2��Ӧ����Ӧʽ����Ϊ��2Na2O2+2SO2=====2Na2SO3+O2��Ϊ���������һ��װ�ã�����֤Na2O2��SO2�ķ�Ӧ���

��ͼ��װ���������װ�������Ժ�����ҩƷ����������Ũ���ᡣ����������⣺
��1����ȼ�ٴ��ƾ��ƣ����ã��۲쵽�ٴ������������壻�۴�Ӳ�ʲ����ܵĹܱڷ��ȣ�����ҩƷ����ɫ�仯Ϊ��___________ɫ��Ϊ___________ɫ��
��2���ڷ�Ӧ����������ýϼ��IJ���֤����Ӧ������O2��
_______________________________________________________________________________��
��3��������ˮ���ռ����壬��ʲô�����֤���ռ����������Ѳ���SO2��
_______________________________________________________________________________��
��4������Ӧ������ȡӲ�ʲ��������������壬װ���Թ��У���ˮ�ܽ�ʱδ�������壬���֤����������Na2SO3��
_______________________________________________________________________________��
��5������Ӧ������ȡӲ�ʲ��������������壬װ���Թ��У���������������Һ��������ų����ټ����Ȼ�����Һ���а�ɫ�������ɣ��Է�������������ԭ��______________________��

��ͼ��װ���������װ�������Ժ�����ҩƷ����������Ũ���ᡣ����������⣺
��1����ȼ�ٴ��ƾ��ƣ����ã��۲쵽�ٴ������������壻�۴�Ӳ�ʲ����ܵĹܱڷ��ȣ�����ҩƷ����ɫ�仯Ϊ��___________ɫ��Ϊ___________ɫ��
��2���ڷ�Ӧ����������ýϼ��IJ���֤����Ӧ������O2��
_______________________________________________________________________________��
��3��������ˮ���ռ����壬��ʲô�����֤���ռ����������Ѳ���SO2��
_______________________________________________________________________________��
��4������Ӧ������ȡӲ�ʲ��������������壬װ���Թ��У���ˮ�ܽ�ʱδ�������壬���֤����������Na2SO3��
_______________________________________________________________________________��
��5������Ӧ������ȡӲ�ʲ��������������壬װ���Թ��У���������������Һ��������ų����ټ����Ȼ�����Һ���а�ɫ�������ɣ��Է�������������ԭ��______________________��
(1)���� �� (2)�ô����ǵ�ľ���������ˮ����Ϸ�����ľ����ȼ����֤����Ӧ������O2 (3)Ʒ����Һ�ĺ�ɫ����ȥ ��4���������ᣬ���д̼�����ζ�������ݳ�����֤�������к���Na2SO3 (5)Na2O2+SO2====Na2SO4��Na2SO4+BaCl2====BaSO4��+2NaCl
Na2O2Ϊ����ɫ���壬��Ӧ�����ɵ�Na2SO3��Na2SO4��Ϊ��ɫ���塣����O2�ķ������ô��л��ǵ�ľ��������SO2�ķ�������Ʒ����Һ������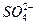 �ķ���ͨ��Ϊ�����ᣬ��������������ʣ�����
�ķ���ͨ��Ϊ�����ᣬ��������������ʣ�����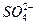 �ķ�������Ba2+����Ҫע���ų�ijЩ���ӵĸ��š�
�ķ�������Ba2+����Ҫע���ų�ijЩ���ӵĸ��š�
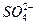 �ķ���ͨ��Ϊ�����ᣬ��������������ʣ�����
�ķ���ͨ��Ϊ�����ᣬ��������������ʣ�����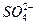 �ķ�������Ba2+����Ҫע���ų�ijЩ���ӵĸ��š�
�ķ�������Ba2+����Ҫע���ų�ijЩ���ӵĸ��š�
��ϰ��ϵ�д�
�����Ŀ

 �ѳ�����������________________________________��
�ѳ�����������________________________________�� �Թ�B
�Թ�B