��Ŀ����
�������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����
(1)Cr�ĺ�������Ų�ʽΪ____________________��
(2)��ѧ��ͨ��X-���߲�õ����Ľṹʾ��ͼ�ɼ�ʾ���£�
(1)Cr�ĺ�������Ų�ʽΪ____________________��
(2)��ѧ��ͨ��X-���߲�õ����Ľṹʾ��ͼ�ɼ�ʾ���£�

ͼ�����߱�ʾ��������Ϊ_______________��
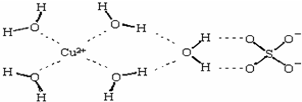
(3)������Һ�백ˮ��һ�������¿�������Cu(NH3)4SO4��H2O���塣
��Cu(NH3)4SO4��H2O�����У�[Cu(NH3)4]2+Ϊƽ�������νṹ�������������ṹ��ԭ������__________��������ԭ�ӵ��ӻ�������______��
(4)����������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡����Ʋ�Ni(CO)4�ľ���������________________��Ni(CO)4����������_________��
A.ˮ
B.���Ȼ�̼
C.��
D.��������Һ
(3)������Һ�백ˮ��һ�������¿�������Cu(NH3)4SO4��H2O���塣
��Cu(NH3)4SO4��H2O�����У�[Cu(NH3)4]2+Ϊƽ�������νṹ�������������ṹ��ԭ������__________��������ԭ�ӵ��ӻ�������______��
(4)����������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡����Ʋ�Ni(CO)4�ľ���������________________��Ni(CO)4����������_________��
A.ˮ
B.���Ȼ�̼
C.��
D.��������Һ
(1)1s22s22p63s23p63d54s1
(2)��������
(3)SO42-��sp3
(4)���Ӿ��壻BC
(2)��������
(3)SO42-��sp3
(4)���Ӿ��壻BC

��ϰ��ϵ�д�
�����Ŀ

 �������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����
�������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����