��Ŀ����
��ɫ��ĩA��NaHCO3��KHCO3�Ļ���ȡ�ס��ҡ���3��������ͬ��A��Ʒ�ֱ���50.0ml��ͬŨ�ȵ������ַ�Ӧ���õ�������������״������A��������ϵ���±����Լ��㣺| ʵ����� | �� | �� | �� |
| ϡ����������mL�� | 50.0 | 50.0 | 50.0 |
| A��Ʒ������g�� | 2.84 | 5.25 | 7.00 |
| ����������L�� | 0.672 | 0.896 | 0.896 |
��2���������NaHCO3���������������������ȷ��0.1%��
���𰸡��������ס��ҡ�������������������ʵ�����ͬ���ס����������Ʒ�������������ɶ�����̼��������ʼ����������������Ʒ��ȫ��Ӧ���ҡ������飬���������������ɶ�����̼��������䣬������������ȫ��Ӧ��������Ʒ��ʣ�࣮����0.896L������̼��Ҫ��Ʒ������Ϊ2.84g× =3.79g����������Ʒ��ʣ�࣬
=3.79g����������Ʒ��ʣ�࣬
��1�����ñ������ݼ��㣬����n=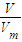 ���������̼�����ʵ���������HCl��CO2����n��HCl�����ٸ���c=
���������̼�����ʵ���������HCl��CO2����n��HCl�����ٸ���c=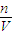 ���㣻
���㣻
��2�����ü������ݼ��㣬��������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�ͼ�������̼������з���ʽ����x��y��ֵ���ٸ���m=nM����NaHCO3����������������NaHCO3������������
����⣺�ס��ҡ�������������������ʵ�����ͬ���ס����������Ʒ�������������ɶ�����̼��������ʼ����������������Ʒ��ȫ��Ӧ���ҡ������飬���������������ɶ�����̼��������䣬������������ȫ��Ӧ��������Ʒ��ʣ�࣮����0.896L������̼��Ҫ��Ʒ������Ϊ2.84g×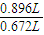 =3.79g����������Ʒ��ʣ�࣬
=3.79g����������Ʒ��ʣ�࣬
��1�����ñ������ݼ��㣬������̼�����ʵ���Ϊ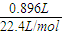 =0.04mol������HCl��CO2��֪n��HCl��=n��CO2��=0.04mol���ʸ���������ʵ���Ũ��Ϊ
=0.04mol������HCl��CO2��֪n��HCl��=n��CO2��=0.04mol���ʸ���������ʵ���Ũ��Ϊ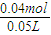 =0.8mol/L��
=0.8mol/L��
�𣺸���������ʵ���Ũ��Ϊ0.8mol/L��
��2�����ü������ݼ��㣬��������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol����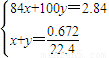
���x=0.01��y=0.02
��NaHCO3������Ϊ0.01mol×84g/mol=0.84g����NaHCO3����������Ϊ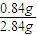 ×100%=29.6%
×100%=29.6%
�𣺻������NaHCO3����������Ϊ29.6%��
���������⿼������ļ��㣬��Ŀ�Ѷ��еȣ�����ע��Ա������ݵķ���������ѧ�������ݷ����������ж����ʹ�������ǽ���Ĺؼ���
 =3.79g����������Ʒ��ʣ�࣬
=3.79g����������Ʒ��ʣ�࣬��1�����ñ������ݼ��㣬����n=
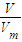 ���������̼�����ʵ���������HCl��CO2����n��HCl�����ٸ���c=
���������̼�����ʵ���������HCl��CO2����n��HCl�����ٸ���c=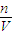 ���㣻
���㣻��2�����ü������ݼ��㣬��������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol�����ݶ�������֮�ͼ�������̼������з���ʽ����x��y��ֵ���ٸ���m=nM����NaHCO3����������������NaHCO3������������
����⣺�ס��ҡ�������������������ʵ�����ͬ���ס����������Ʒ�������������ɶ�����̼��������ʼ����������������Ʒ��ȫ��Ӧ���ҡ������飬���������������ɶ�����̼��������䣬������������ȫ��Ӧ��������Ʒ��ʣ�࣮����0.896L������̼��Ҫ��Ʒ������Ϊ2.84g×
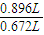 =3.79g����������Ʒ��ʣ�࣬
=3.79g����������Ʒ��ʣ�࣬��1�����ñ������ݼ��㣬������̼�����ʵ���Ϊ
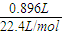 =0.04mol������HCl��CO2��֪n��HCl��=n��CO2��=0.04mol���ʸ���������ʵ���Ũ��Ϊ
=0.04mol������HCl��CO2��֪n��HCl��=n��CO2��=0.04mol���ʸ���������ʵ���Ũ��Ϊ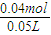 =0.8mol/L��
=0.8mol/L���𣺸���������ʵ���Ũ��Ϊ0.8mol/L��
��2�����ü������ݼ��㣬��������NaHCO3��KHCO3�����ʵ����ֱ�Ϊxmol��ymol����
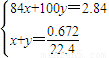
���x=0.01��y=0.02
��NaHCO3������Ϊ0.01mol×84g/mol=0.84g����NaHCO3����������Ϊ
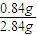 ×100%=29.6%
×100%=29.6%�𣺻������NaHCO3����������Ϊ29.6%��
���������⿼������ļ��㣬��Ŀ�Ѷ��еȣ�����ע��Ա������ݵķ���������ѧ�������ݷ����������ж����ʹ�������ǽ���Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
| |||||||||||
��ɫ��ĩA��NaHCO3��KHCO3�Ļ���ȡ�ס��ҡ���3��������ͬ��A��Ʒ�ֱ���50.0ml��ͬŨ�ȵ������ַ�Ӧ���õ�������������״������A��������ϵ���±����Լ��㣺
| ʵ����� | �� | �� | �� |
| ϡ����������mL�� | 50.0 | 50.0 | 50.0 |
| A��Ʒ������g�� | 2.84 | 5.25 | 7.00 |
| ����������L�� | 0.672 | 0.896 | 0.896 |
��2���������NaHCO3���������������������ȷ��0.1%��
