��Ŀ����
����Ҫ��д�����з�Ӧ���Ȼ�ѧ����ʽ
(1)һ����������������Ӧ�����Ȼ�������,������1mol���ȼ�ʱ�ų�91.5kJ������________________________________________________________.
(2)ij��ѧ��Ӧ�������仯��ͼ��ʾ,�÷�Ӧ���Ȼ�ѧ����ʽ��(��Ӧ����abc��ʾ)
_____________________________________________.
(3)ij��Ӧ��ƽ�ⳣ��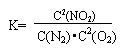 �����1molN2��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
�����1molN2��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
(4)ʵ���в���ֱ�Ӳ��ʯī���������ɼ��鷴Ӧ�ķ�Ӧ��,���ɲ�����顢ʯī������ȼ�յķ�Ӧ��:
CH4(g)+2O2(g)=CO2(g)+2H2O(l)����H1=-890.3kJ/mol
C(ʯī,s)+O2(g)=CO2(g) ����H2="-393.5" kJ/mol
H2(g)+1/2O2(g)=H2O(l) ����H3="-285.8" kJ/moL
����ʯī��������Ӧ���ɼ�����Ȼ�ѧ��Ӧ����ʽΪ__________________________________________.

����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 �����1molN2 ��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.
�����1molN2 ��ȫ��Ӧ,Ҫ��������68kJ.д���÷�Ӧ���Ȼ�ѧ����ʽ______________________________________________.