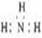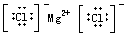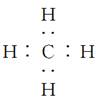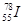��Ŀ����
��100 mL NaOH��Һ��ͨ��CO2��ַ�Ӧ���ڼ�ѹ�ͽϵ��¶��£�С�ĵؽ���Һ���ɣ��õ���ɫ����M��ͨ�������̼�����V(��״����)��M������W�Ĺ�ϵ��ͼ
��ʾ����ش��������⣺
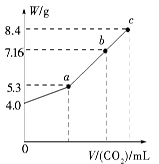
(1)b��ʱM����ɳɷ�Ϊ______________________��
(2)��Ҫʹb�����ɵ��ε�������Ϊ8.4 g����Ӧ��������Һ��ͨ�������̼________L(��״����)��
(3)�������ɵ�7.16 g�ε���Һ�м���һ������ij���ʣ���ַ�Ӧ��ѹ���������õ�������̼���ƹ���(�ᾧˮ)8.4 g��
����ֻ����0.03 molij���ʣ����������ʿ�����________��________��
����ֻ����0.06 molij���ʣ����������ʿ�����________��________��________��
(4)�����£�ͬŨ�ȵ�̼������Һ��̼��������Һ��pH
������7��������________��pH����������________________________��0.1 mol��L��1̼������Һ������Ũ�ȵĴ�С��ϵ��________����̼��������Һ����ε�������������Һ��������Ӧ�����ӷ���ʽΪ________��
��ʾ����ش��������⣺

(1)b��ʱM����ɳɷ�Ϊ______________________��
(2)��Ҫʹb�����ɵ��ε�������Ϊ8.4 g����Ӧ��������Һ��ͨ�������̼________L(��״����)��
(3)�������ɵ�7.16 g�ε���Һ�м���һ������ij���ʣ���ַ�Ӧ��ѹ���������õ�������̼���ƹ���(�ᾧˮ)8.4 g��
����ֻ����0.03 molij���ʣ����������ʿ�����________��________��
����ֻ����0.06 molij���ʣ����������ʿ�����________��________��________��
(4)�����£�ͬŨ�ȵ�̼������Һ��̼��������Һ��pH
������7��������________��pH����������________________________��0.1 mol��L��1̼������Һ������Ũ�ȵĴ�С��ϵ��________����̼��������Һ����ε�������������Һ��������Ӧ�����ӷ���ʽΪ________��
(1)Na2CO3��NaHCO3��(2)0.448
(3)��Na2O��Na2O2����Na��NaOH��NaH
(4)Na2CO3������ͬ�����£�CO32����ˮ��������HCO3����ˮ������ǿ��c(Na��)��c(CO32��)��c(OH��)��c(HCO3��)��c(H��)��Ba2����2OH����2HCO3��=BaCO3����CO32����2H2O
(3)��Na2O��Na2O2����Na��NaOH��NaH
(4)Na2CO3������ͬ�����£�CO32����ˮ��������HCO3����ˮ������ǿ��c(Na��)��c(CO32��)��c(OH��)��c(HCO3��)��c(H��)��Ba2����2OH����2HCO3��=BaCO3����CO32����2H2O
(1)��ͼ��֪����ʼʱ��������Ϊ4.0 g�����ʵ���Ϊ0.1 mol����ȫ������̼���ƣ���WΪ5.3 g����ȫ������̼�����ƣ���WΪ8.4 g��b���Ӧ�Ĺ���MΪ7.16 g������5.3 g��8.4 g֮�䡣��b���Ӧ�Ĺ���Ϊ̼���ƺ�̼�����ƵĻ���(2)��b���Ӧ�Ĺ�����̼���ơ�̼�����Ƶ����ʵ����ֱ�Ϊx��y������106 g��mol��1��x��84 g��mol��1��y��7.16 g,2x��y��0.1 mol����ã�x��0.02 mol��y��0.06 mol����Na2CO3��H2O��CO2=2NaHCO3�ã�n(CO2)��0.02 mol��(3)8.4 g Na2CO3�����ʵ���Ϊ0.08 mol�������⣬0.06 mol NaHCO3�D��0.06 mol Na2CO3�������ķ�ӦΪNaHCO3��NaOH=Na2CO3��H2O��n(NaOH)��0.06 mol������ˮ�����������Ƶ������У��ơ��������ơ������ơ��⻯��(NaH��H2O=NaOH��H2��)�����������ʡ�

��ϰ��ϵ�д�
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
�����Ŀ