��Ŀ����
����ѧ����ѡ���л���ѧ������
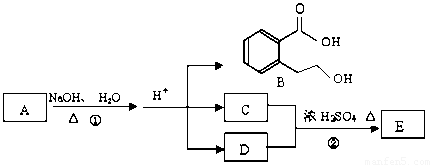
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���úͣĵ���Է���������ȣ���EΪ��֧���Ļ����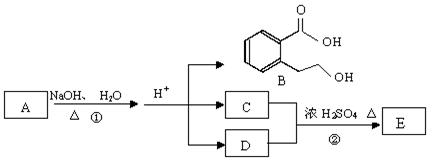
������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ������E�ķ���ʽΪ ��
��2��������B���ܷ����ķ�Ӧ�� ������ĸ��ţ���
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ d��������Ӧ e��ˮ�ⷴӦ f���û���Ӧ
��3����Ӧ�ڵĻ�ѧ����ʽ�� ��
��4��C�����еĹ����������� ��A�Ľṹ��ʽ�� ��
��5��ͬʱ������������������B��ͬ���칹�����Ŀ�� ����
���м��ȡ�������ṹ �����ڷǷ������� ���� FeCl3��Һ������ɫ��Ӧ��д����������һ��ͬ���칹��Ľṹ��ʽ ��
��15�֣���1��C5H10O2 ��2�֣� ��2��e ��2�֣�
��3��CH3COOH + CH3CH2CH2OH CH3COOCH2CH2CH3 + H2O��2�֣�
CH3COOCH2CH2CH3 + H2O��2�֣�
��4���Ȼ���1�֣���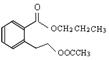 ��
��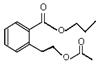 ��2�֣�
��2�֣�
��5��4��2�֣�  д������֮һ���ɣ�2�֣�
д������֮һ���ɣ�2�֣�
���������������1��E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%����̼��ԭ�ӵĸ����ֱ���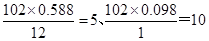 ������ԭ�ӵĸ�����
������ԭ�ӵĸ����� ������E�ķ���ʽΪC5H10O2��
������E�ķ���ʽΪC5H10O2��
��2��������B�����к����Ȼ����ǻ��ͱ��������Բ����ܷ���ˮ�ⷴӦ����ѡe��
��3��CD��A��ˮ�����úͣĵ���Է���������ȣ���CD����E�����Ը���E�Ļ�ѧʽ��֪��C��D�ֱ�������ͱ�������÷�Ӧ�Ļ�ѧ����ʽ��CH3COOH + CH3CH2CH2OH CH3COOCH2CH2CH3 + H2O��
CH3COOCH2CH2CH3 + H2O��
��4��C�����еĹ������������Ȼ�������BCD�Ľṹ��ʽ��֪��A�Ľṹ��ʽ��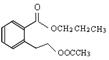 ��
��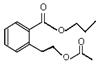 ��
��
��5���� FeCl3��Һ������ɫ��Ӧ��������к��з��ǻ������ڷǷ�����������˵�������ϲ������Ȼ�������Ϊ���м��ȡ�������ṹ�����Կ��ܵĽṹ��ʽ��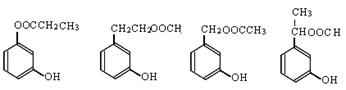 ��
��
���㣺�����л���ṹ��ʽ����ѧʽ�������Լ�ͬ���칹����жϺͷ���ʽ����д
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿����ۺ���ǿ����ע�ضԻ���֪ʶ������ѵ����ͬʱ�����ض�ѧ����������������ⷽ����ָ����ѵ�������������ܽ�ȫ��ؿ���ѧ�����л���ѧ����֪ʶ����˼ά����������˼ά���������ѧ����Ӧ������������Ĺؼ��Ǽ�ס���������ŵĽṹ�������Լ��֮���ת����Ȼ��������ü��ɡ�