��Ŀ����
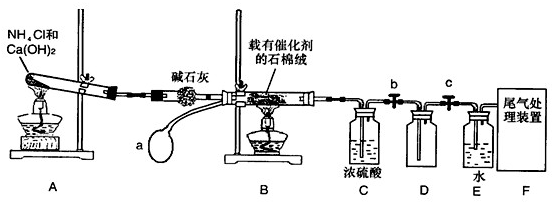
ij��ѧ����С��ģ�ҵ������ȡHNO3�������ͼ��ʾװ�ã�����aΪһ���ɳ��������������Ƥ����ش���������:
(1)д��װ��A����Ҫ��Ӧ�Ļ�ѧ����ʽ
(2)��μ��װ��A��������
(3)��֪1molNO2��Һ̬ˮ��Ӧ����HNO3��Һ��NO����ų�����45.5kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ
�÷�Ӧ��һ�����淴Ӧ����Ҫ���NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��________
A�������¶� B�������¶� C������ѹǿ D������ѹǿ
(4)ʵ������ر�ֹˮ��b��c����װ��D�������ˮ�У�������______
(5)װ��C��ŨH2SO4��������
(6)��������û�ѧС�����ʵ������ȡNH3����һ���� (�û�ѧ��Ӧ����ʽ��ʾ)
(1)2NH4Cl+Ca(OH)2![]() 2NH3��+CaCl2+2H2O(2��)
2NH3��+CaCl2+2H2O(2��)
��2����A�е����¶˽���ˮ�У���˫�ֽ��մ��Թܵײ������ܿ������ݲ�����˫���뿪�����ܲ���һ��ˮ����˵��װ��A��©����(2��)
(3)3NO2(g)+H2O(l)=2HNO3(aq)+NO(g) ��H=��136.5kJ��mol-1(2��)BC (2��)
(4)��ɫ��dz��(2��)(5)���ն����NH3��(2��)
![]() (6) NH3��H2O = NH3��+H2O�� (����������Ҳ����) (2��)
(6) NH3��H2O = NH3��+H2O�� (����������Ҳ����) (2��)
����:
��

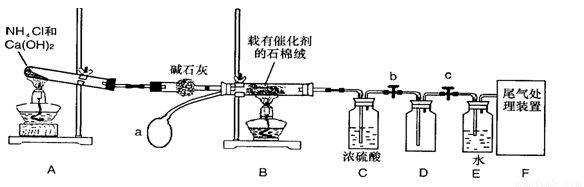
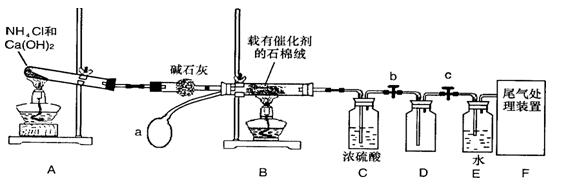
ij��ѧ����С��ģ�ҵ������ȡHNO3�������ͼ��ʾװ�ã�����aΪһ���ɳ��������������Ƥ����ش���������: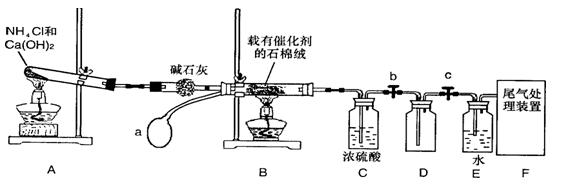
(1)д��װ��A����Ҫ��Ӧ�Ļ�ѧ����ʽ (2��)
(2)��μ��װ��A�������� (2��)
(3)��֪1molNO2��Һ̬ˮ��Ӧ����HNO3��Һ��NO����ų�����45.5kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ (2��)
�÷�Ӧ��һ�����淴Ӧ����Ҫ���NO2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��________(2��)
| A�������¶� | B�������¶� | C������ѹǿ | D������ѹǿ |
(5)װ��C��ŨH2SO4�������� (2��)
(6)��������û�ѧС�����ʵ������ȡNH3����һ���� (�û�ѧ��Ӧ����ʽ��ʾ)��2�֣�