��Ŀ����
����(P)��Cl2������Ӧ����PCl3��PCl5����Ӧ���̺������Ĺ�ϵ����ͼ��ʾ��ͼ�еĦ�H��ʾ����1 mol��������ݡ���֪PCl5�ֽ�����PCl3��Cl2���÷ֽⷴӦ�ǿ��淴Ӧ������˵����ȷ���� (����)
| A�������������䣬�����¶�������PCl5������ |
| B����Ӧ2P(s)��5Cl2(g)=2PCl5(g)��Ӧ�ķ�Ӧ�ȡ���H����798 kJ��mol��1 |
| C��P��Cl2��Ӧ����PCl3���Ȼ�ѧ����ʽΪ2P(s)��3Cl2(g)=2PCl3(g)����H����306 kJ��mol��1 |
| D�������������䣬����PCl5�ֽ�����PCl3��Cl2�ķ�Ӧ������ѹǿ��PCl5��ת���ʼ�С��ƽ�ⳣ��K���� |
BD
����

 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д���ѧ��ӦN2��3H2 2NH3�������仯����ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��
2NH3�������仯����ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ� ��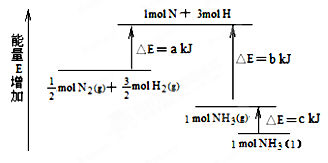
A��N2(g)��3H2(g)  2NH3(l) �SH ��2(a��b��c)kJ��mol��1 2NH3(l) �SH ��2(a��b��c)kJ��mol��1 |
B��N2(g)��3H2(g)  2NH3(g) �SH ��2(b��a)kJ��mol��1 2NH3(g) �SH ��2(b��a)kJ��mol��1 |
C��1/2N2(g)��3/2H2(g)  NH3(l)�SH �� (b��c��a)kJ��mol��1 NH3(l)�SH �� (b��c��a)kJ��mol��1 |
D��1/2N2(g)��3/2H2(g)  NH3(g)�SH �� (a��b)kJ��mol��1 NH3(g)�SH �� (a��b)kJ��mol��1 |
��֪:HCN(aq)��NaOH(aq)��Ӧ����1 molˮ�Ħ�H="-12.1" kJ��mol-1;HCl(aq)��NaOH(aq)��Ӧ����1 molˮ�Ħ�H="-57.3" kJ��mol-1����HCN��ˮ��Һ�е���Ħ�H����(����)
| A��-69.4 kJ��mol-1 | B��-45.2 kJ��mol-1 |
| C��+45.2 kJ��mol-1 | D��+69.4 kJ��mol-1 |
�������ȷ�Ӧ��˵����ȷ����( )��
| A��������ȵķ�Ӧһ�������ȷ�Ӧ |
| B��ֻ�зֽⷴӦ�������ȷ�Ӧ |
| C��ʹ�ô����ķ�Ӧ�����ȷ�Ӧ |
| D��CO2��CaO�����Ƿ��ȷ�Ӧ,��CaCO3�ֽ������ȷ�Ӧ |
ȫ���ů�����������˾��ӣ����������پ����ս������˵������ȷ����(����)
| A���ƹ㡰��̼���á�����������������ŷ� |
| B���ƽ�С��������վ���˽�������ط��õ����ѣ��ٽ��ط����õĿ��ٷ�չ |
| C���ƹ㡰��ɫ���ɡ��ƻ������տ����е�CO2������������Դ�ϳ����� |
| D�����þ����������̫���ܵ�ؿɽ�̫����ֱ��ת��Ϊ���� |
��ҵ����ˮú���ķ�ӦΪ��C(s)��H2O(g)=CO(g)��H2(g)����H����131.4 kJ��mol��1�������ж���ȷ���� (����)��
| A����Ӧ�������ܺʹ��������������ܺ� |
| B��CO(g)��H2(g)=C(s)��H2O(l)��H����131.4 kJ��mol��1 |
| C��ˮú����Ӧ������1 mol H2(g)����131.4 kJ���� |
| D��ˮú����Ӧ������1���CO(g)����131.4 kJ���� |
��֪��2H2(g)��O2(g)=2H2O(l)����H����571.6 kJ��mol��1
2CH3OH(l)��3O2(g)=2CO2(g)��4H2O(l)����H����1452 kJ��mol��1
H��(aq)��OH��(aq)=H2O(l)����H����57.3 kJ��mol��1
����˵����ȷ���� (����)
| A��H2(g)��ȼ����Ϊ571.6 kJ��mol��1 |
| B��ͬ������H2(g)��CH3OH(l)��ȫȼ�գ�H2(g)�ų��������� |
C�� H2SO4(aq)�� H2SO4(aq)�� Ba(OH)2(aq)= Ba(OH)2(aq)= BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 BaSO4(s)��H2O(l)����H����57.3 kJ��mol��1 |
| D��3H2(g)��CO2(g)=CH3OH(l)��H2O(l)����H����135.9 kJ��mol��1 |
���������������ֵ�̼�������(����)��
| A������ʳ��ӹ����� |
| B��ע���Լ�õ� |
| C���������صġ�������ʳ�� |
| D������ʹ��н��Ϊȼ�� |
�йؼ������������ʾ��
| ��ѧ�� | N��N | H��H | H��N |
| ����/kJ��mol��1 | x | 436 | 391 |
A��945.6������B��649������C��431������D��869