��Ŀ����
��֪��1��HCl��NaOH��Ӧ���к��Ȧ�H����57.3kJ/mol��
��2��CO��g����ȼ������283.0kJ/mol��������������ȷ���ǣ� ��
A��H2SO4��NaOH��Ӧ���к��Ȧ�H��2������57.3��kJ/mol
B��2CO2��g�� ��2CO��g����O2��g������H��+2��283.0kJ/mol
C��1/2H2SO4��Ũ��+NaOH��aq��=1/2Na2SO4��aq��+H2O��1���� ��H=��57.3kJ/mol
D��1mol����ȼ�����ų��������Ǽ����ȼ����
B

��ϰ��ϵ�д�
 ���Ǽ���С����ϵ�д�
���Ǽ���С����ϵ�д� �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д�
�����Ŀ


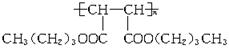
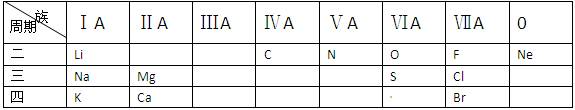
 ������ ��
������ �� ��)��
��)�� 2H2O����Cl2��H2��ϣ��ڹ����¾Ϳ��Է�����ը������HCl���ݴ��ƶϣ������ԱȽϣ�O2 Cl2 (����/С��)��
2H2O����Cl2��H2��ϣ��ڹ����¾Ϳ��Է�����ը������HCl���ݴ��ƶϣ������ԱȽϣ�O2 Cl2 (����/С��)��