题目内容
用一氧化碳和氢气在443—473 K温度下,并用钴作催化剂时,可以反应生成
n=5—8的烷烃,这是人工合成汽油的方法之一。
(1)写出用CnH2n+2表示的人工合成汽油的配平的化学方程式_____________________。
(2)如果向密闭的合成塔里通入恰好能完全反应的CO和H2,当完全反应后,合成塔内温度不变,而塔内气体压强降低到原来的2/5。通过计算说明这时有无汽油生成。
(3)要达到上述合成汽油的要求,CO和H2的体积比的取值范围是多少?
n=5—8的烷烃,这是人工合成汽油的方法之一。
(1)写出用CnH2n+2表示的人工合成汽油的配平的化学方程式_____________________。
(2)如果向密闭的合成塔里通入恰好能完全反应的CO和H2,当完全反应后,合成塔内温度不变,而塔内气体压强降低到原来的2/5。通过计算说明这时有无汽油生成。
(3)要达到上述合成汽油的要求,CO和H2的体积比的取值范围是多少?
(1)nCO+(2n+1)H2 CnH2n+2+nH2O
CnH2n+2+nH2O
(2)无汽油生成
(3)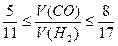
 CnH2n+2+nH2O
CnH2n+2+nH2O(2)无汽油生成
(3)
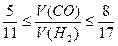
(1)二者反应的化学方程式为
nCO+(2n+1)H2 CnH2n+2+nH2O
CnH2n+2+nH2O
(2)由压强比等于物质的量比可得
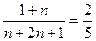 n=3
n=3
汽油所含碳原子数为C5—C11。
所以,无汽油生成。
(3)若n=5
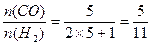 ;
;
若n=8
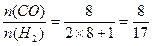 。
。
所以,CO和H2的体积比的取值范围是
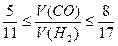 。
。
nCO+(2n+1)H2
 CnH2n+2+nH2O
CnH2n+2+nH2O(2)由压强比等于物质的量比可得
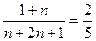 n=3
n=3汽油所含碳原子数为C5—C11。
所以,无汽油生成。
(3)若n=5
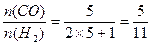 ;
;若n=8
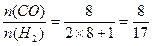 。
。所以,CO和H2的体积比的取值范围是
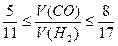 。
。
练习册系列答案
相关题目