��Ŀ����
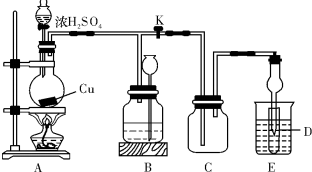
��13�֣�ij��ѧ��ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ������ͼ��ʾװ�ý����й�ʵ�顣��ش�
I��ʵ���м�ͬѧȡa �� Cu Ƭ��12ml 18mol/LŨH2SO4����Բ����ƿ�м��ȣ�ֱ����Ӧ��ϣ��������ƿ�л���һ������H2SO4��Cuʣ�ࡣ
��1����д��Cu��ŨH2SO4��Ӧ�Ļ�ѧ����ʽ�� ��
װ��E���Թ�D��ʢƷ����Һ����C�����弯����D���п��ܹ۲쵽��������__________��
ʵ��װ��D�п�����ɻ�����Ⱦ��������������Խ����ʵ����Ʒ��ѡ��_________��
��2��װ��B�������������������塣��D������������ر�����K����ȥ�ƾ��ƣ����������ȵ����ã�A�����������������B��B��Ӧ���õ�Һ�壨����ĸ����________
A������Na2SO3��Һ B������ KMnO4��Һ
C��Ũ��ˮ D������NaHSO3��Һ
��3���������ۣ�Ϊʲô��һ���������ᵫδ��ʹCu��ȫ�ܽ���?
��������ҩƷ�ܹ�����֤����Ӧ���������ƿ�е�ȷ���������_________��
A��Fe�� B��BaCl2��Һ C��Ag D������NaHSO3��Һ
��4��ʵ���м�ѧ����A�з�Ӧ����Һ��ͨ��һ�ֳ������嵥�ʣ�ʹͭƬȫ���ܽ��ҽ���������ͭ��Һ�����ʸ����嵥����___ �������ƣ�����Ӧ����ʽ��______ ��
��1�� Cu��2H2SO4��Ũ�� CuSO4��SO2�� +2H2O �� Ʒ����Һ��ɫ ��
CuSO4��SO2�� +2H2O �� Ʒ����Һ��ɫ ��
�ý���NaOH��Һ�����������Թܿ� ��2��D ���˿�1�֣�
��3��AD ��4������ �� 2Cu��2H2SO4 +O2��2CuSO4��2H2O
����

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�