��Ŀ����
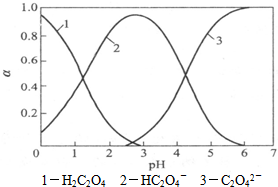 ���ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ�����ֽܷ⣬����ˮ��Һ�д�����ʽ�ķֲ���pH��ϵ��ͼ��ʾ��
���ᣨH2C2O4����һ�ֶ�Ԫ���ᣬ�����ֽܷ⣬����ˮ��Һ�д�����ʽ�ķֲ���pH��ϵ��ͼ��ʾ����1����д��pH��3����5�Ĺ����з�Ӧ�����ӷ���ʽ��
HC2O4-+OH-=C2O42-+H2O
HC2O4-+OH-=C2O42-+H2O
��2��H2C2O4��Һ�У�c��H+��/c��C2O42-��
��
��
2�����������������=��������3��H2C2O4�����ֽܷ⣬�ֽⷽ��ʽΪ��
H2C2O4
CO��+CO2��+H2O
| ||
H2C2O4
CO��+CO2��+H2O
| ||
��4��H2C2O4�DZ궨KMnO4��ҺŨ�ȳ��õĻ����ʣ��궨KMnO4��ҺŨ��ʱ��Ӧ����Һ������75��85�棬�¶ȵ��ڻ���ڸ÷�Χʱ�������ڵζ������ȷ��������_________��
��5���±�Ϊ���ʵ���Ũ����ͬ�ļ���������⼰��¯ˮ�����ܽ��ԵıȽϣ�
| �� | ����[Fe��OH��3]�ܽ���/g | ˮ����CaCO3���ܽ���/g |
| ���� | 0.7911 | ȫ�� |
| ���� | 0.1586 | �� |
| ���� | 0.7399 | 0.0036 |
| ���� | 0.1788 | 0.1655 |
C
C
������ĸ����A������Խǿ���������Ч��Խ��
B�������ˮ��Ч�����ԭ������Ϊ��������̫��
C�������ˮ�������е��ܽ�����������ӵ����ʿ����й�
D������������ˮ����Ч�����ã�
��������1������ͼ���У�����Ũ�ȱ仯ȷ�����������ӷ�Ӧ��
��2������ĵ�һ������̶Ƚϴڶ�������̶Ƚ�С��
��3�����������ֽ����ɶ�����̼��һ����̼��ˮ��
��4�����ݷ�Ӧ���ʺͲ�������ʷ�����
��5��A����������ǿ�ᣬ�����Լ����������ᣬ���ݱ��е��������ش�
B��C2O42-��Ca2+�γɳ�����ֹˮ�����ܽ⣻
C�������ˮ�������е��ܽ�����������ӵ����ʿ����йأ�
D�������ˮ����Ӧ���ɵ���������ܵģ������ڳ�����
��2������ĵ�һ������̶Ƚϴڶ�������̶Ƚ�С��
��3�����������ֽ����ɶ�����̼��һ����̼��ˮ��
��4�����ݷ�Ӧ���ʺͲ�������ʷ�����
��5��A����������ǿ�ᣬ�����Լ����������ᣬ���ݱ��е��������ش�
B��C2O42-��Ca2+�γɳ�����ֹˮ�����ܽ⣻
C�������ˮ�������е��ܽ�����������ӵ����ʿ����йأ�
D�������ˮ����Ӧ���ɵ���������ܵģ������ڳ�����
����⣺��1������ͼ��֪��pH��3����5�Ĺ����У�HC2O4-Ũ�Ƚ�С��C2O42-Ũ��������˵��HC2O4-�����������ӷ����кͷ�Ӧ�����Ը����ӷ�Ӧ����ʽΪ��HC2O4-+OH-=C2O42-+H2O���ʴ�Ϊ��HC2O4-+OH-=C2O42-+H2O��
��2�������Ƕ�Ԫ���ᣬ��һ������̶ȱȵڶ�������̶ȴڶ����������ɵ�c��H+��=c��C2O42-������һ���������c��H+��ԶԶ���ڵڶ����������c��H+��������
��2���ʴ�Ϊ������
��3�����������£�����ֽ�����һ����̼��������̼��ˮ����Ӧ����ʽΪ��H2C2O4
CO��+CO2��+H2O���ʴ�Ϊ��
H2C2O4
CO��+CO2��+H2O��
��4������¶Ƚϵͣ���Ӧ���ʽ�������ɫ�仯�����ԣ�����¶ȹ��ߣ����Ჿ�ַֽ�Ӱ���⣬����Ӧ����Һ������75��85�棬�¶ȵ��ڻ���ڸ÷�Χʱ�������ڵζ������ȷ��
�ʴ�Ϊ���¶ȹ��ͷ�Ӧ���ʻ������¶ȹ���ʱ��H2C2O4��������Һ�лᲿ�ַֽ⣻
��5��A����������ǿ�ᣬ�����Լ����������ᣬ���ݱ��е����ݣ�ͬ���ʵ���Ũ��ʱ������Խǿ�����������Ч��������ᣬ��A����
B�������ˮ��Ч�����ԭ����C2O42-��Ca2+�γɳ�����ֹˮ�����ܽ⣬������Ӧ���ʣ���B����
C�������ˮ�������е��ܽ�����������ӵ����ʿ����йأ���C��ȷ��
D�������ˮ����Ӧ���ɵ���������ܵģ���ֹˮ�����ܽ⣬�����ڳ�������D����
��ѡC��
��2�������Ƕ�Ԫ���ᣬ��һ������̶ȱȵڶ�������̶ȴڶ����������ɵ�c��H+��=c��C2O42-������һ���������c��H+��ԶԶ���ڵڶ����������c��H+��������
| C(H+) |
| C(C2O42-) |
��3�����������£�����ֽ�����һ����̼��������̼��ˮ����Ӧ����ʽΪ��H2C2O4
| ||
H2C2O4
| ||
��4������¶Ƚϵͣ���Ӧ���ʽ�������ɫ�仯�����ԣ�����¶ȹ��ߣ����Ჿ�ַֽ�Ӱ���⣬����Ӧ����Һ������75��85�棬�¶ȵ��ڻ���ڸ÷�Χʱ�������ڵζ������ȷ��
�ʴ�Ϊ���¶ȹ��ͷ�Ӧ���ʻ������¶ȹ���ʱ��H2C2O4��������Һ�лᲿ�ַֽ⣻
��5��A����������ǿ�ᣬ�����Լ����������ᣬ���ݱ��е����ݣ�ͬ���ʵ���Ũ��ʱ������Խǿ�����������Ч��������ᣬ��A����
B�������ˮ��Ч�����ԭ����C2O42-��Ca2+�γɳ�����ֹˮ�����ܽ⣬������Ӧ���ʣ���B����
C�������ˮ�������е��ܽ�����������ӵ����ʿ����йأ���C��ȷ��
D�������ˮ����Ӧ���ɵ���������ܵģ���ֹˮ�����ܽ⣬�����ڳ�������D����
��ѡC��
���������⿼����������ʵĵ��롢��ѧ��Ӧ����ʽ����д��֪ʶ�㣬�����ͼ���ǽⱾ��Ĺؼ����ѶȽϴ�

��ϰ��ϵ�д�
�����Ŀ



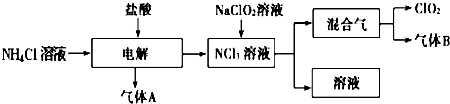
 �ö������ȣ�ClO2�����������ƣ�Na2FeO4Ħ������Ϊ166g?mol-1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl-��Fe3+��
�ö������ȣ�ClO2�����������ƣ�Na2FeO4Ħ������Ϊ166g?mol-1�������;�ˮ�������ͳ�ľ�ˮ��Cl2�Ե�ˮ���������dz�������ˮ�����¼�����ClO2��Na2FeO4��ˮ���������зֱ𱻻�ԭΪCl-��Fe3+��