��Ŀ����
��֪ϡ������ͭ��Ӧʱ��NO3- һ�㱻��ԭΪNO��Ũ������ͭ��Ӧʱ��NO3- һ�㱻��ԭΪNO2���ֽ�25.6gCuͶ�뵽50mL12mol/L��Ũ�����У���ַ�Ӧ���ռ���4.48L�������NO��NO2�Ļ�����塣
��1��д��ͭ�ֱ���Ũ�����ϡ���ᷴӦ�Ļ�ѧ����ʽ��______________________________________________________________��
_______________________________________________________________��
��2������ԭ����������ʵ���Ϊ��____________
��3��25.6gCu��50mL12mol/L��Ũ���ᷴӦ��ʣ����Ϊ��____________�������ʵ���Ϊ��____________��
��1��д��ͭ�ֱ���Ũ�����ϡ���ᷴӦ�Ļ�ѧ����ʽ��______________________________________________________________��
_______________________________________________________________��
��2������ԭ����������ʵ���Ϊ��____________
��3��25.6gCu��50mL12mol/L��Ũ���ᷴӦ��ʣ����Ϊ��____________�������ʵ���Ϊ��____________��
��1��Cu+4HNO3��Ũ����Cu(NO3)2+2NO2��+2H2O
3Cu+8HNO3��ϡ����3 Cu(NO3)2+2NO��+4H2O
��2��0.2mol ��3��ͭ�� 0.2mol
3Cu+8HNO3��ϡ����3 Cu(NO3)2+2NO��+4H2O
��2��0.2mol ��3��ͭ�� 0.2mol
�����������1���������ǿ�����ԣ��ͽ���ͭ��Ӧ�Ļ�ѧ����ʽ�ֱ���Cu��4HNO3��Ũ����Cu(NO3)2��2NO2����2H2O��3Cu��8HNO3��ϡ����3 Cu(NO3)2��2NO����4H2O��
��2�����ᱻ��ԭ����NO��NO2�����Ա���ԭ����������ʵ����������ɵĻ���������ʵ�������
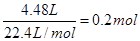 ��
����3�����ݣ�2����֪��û�б���ԭ����������ʵ�����
0.05L��12mol/L��0.2mol��0.4mol
25.6gͭ�����ʵ�����25.6g��64g/mol��0.4mol
���ͭ��ȫ��Ӧ��Ӧ������0.4mol����ͭ
��û�б���ԭ����������ʵ�������0.8mol��0.4mol
��˵����Ӧ�������Dz���ģ�ͭ����
Ӧ����������ͭ�����ʵ�����0.4mol��2��0.2mol��0.2mol
���Թ�����ͭ�����ʵ�����0.4mol��0.2mol��0.2mol
�����������������е��Ѷȵļ����⣬����Ĺؼ���ѧ�����úø����غ㷨���⣬��ѧ�����г��õ��غ㷨���������غ㶨�ɡ�ԭ���غ㡢���ӵĵ�ʧ�غ��Լ�����غ�ȣ���Ҫѧ����ƽʱ��ѵ��ע���ܽᡢ���ɺͻ��ۡ�

��ϰ��ϵ�д�
�����Ŀ
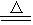 MnCl2��Cl2�� �� 2H2O��
MnCl2��Cl2�� �� 2H2O�� TiCl4��2CO������ TiCl4��2Mg
TiCl4��2CO������ TiCl4��2Mg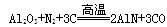 ������������ȷ���� ( )
������������ȷ���� ( ) Mn��CN��2 + ��CN��2�� + 2H2O
Mn��CN��2 + ��CN��2�� + 2H2O