��Ŀ����
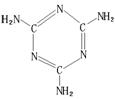
��1�������谷(�ṹ����ͼ)�������京�����߱�������ũ���ӵ�ţ���� ������ߡ������ʵĺ��������ȫ������Ӥ����ʳ�������̷۶�������ʯ�� 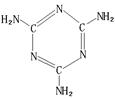
�����谷��Cԭ�ӵ��ӻ���ʽ��
���ֻ�����Nԭ�ӵ��ӻ���ʽ�ֱ��� �� ��
��2������A��һ�ֲ��ȶ������ʣ����ķ���ʽ�ɱ�ʾΪOxFy��10mL A�����ֽܷ��Ϊ15mL O2��10mL F2��ͬ��ͬѹ��
��A�ķ���ʽ�� ��
����֪A�ķ����е�x����ԭ�ӳʡ�O��O��O����״���У���A�ĵ���ʽ�� ��A���ӵĽṹʽ�� ��
��3����CrCl3��ˮ��Һ�У�һ�������´������Ϊ[CrCln(H2O)6-n]x+��n��x��Ϊ�������������ӣ�����ͨ�������ӽ�����֬��R-H�����ɷ������ӽ�����Ӧ��[CrCln(H2O)6-n]x+��x R-H��Rx[CrCln(H2O)6-n]��xH+
����������H+���ⶨ���������x��n,ȷ�������ӵ���ɡ�
����0.0015mol [CrCln(H2O)6-n]x+����Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol/L NaOH��Һ25.00ml,��֪:
x= ,n= , �������ӵĻ�ѧʽΪ ��
(1) sp2 sp2 sp3��ÿ��1�֣�
(2) ��F2O3��1�֣� �� 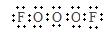 F��O��O��O��F ��ÿ��2�֣�
F��O��O��O��F ��ÿ��2�֣�
(3) 2  1 ��ÿ��1�֣� [CrCl (H2O)5]2+ ��2�֣�
1 ��ÿ��1�֣� [CrCl (H2O)5]2+ ��2�֣�
����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�



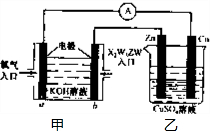 ��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��
��֪X��Y��Z��W���ֶ�����Ԫ�ص�ԭ�Ӱ뾶���μ�С�������ڱ���X��Y��Y��Z��λͬһ���ڵ�����λ�ã�X������������Ϊ������������2����W�ֱ�����X��Y��Z��һ��ԭ�������γɵ�������Ϊ10�ij�����������ж�X��Y��Z��W����Ԫ�ز��ش��������⣺��Ҫ����ȷ�����Ԫ�ط��ż��йػ�ѧ�����ʾ��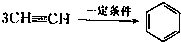 �����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��
�����谷Ҳ�������谷��WXY�����е�Wԭ�ӱ�����ȡ����ɵ��谷��������������Ȳ�����۷�Ӧ���õ��������谷���Ǽ��Լ�������������������д�������谷�ṹ��ʽ��