��Ŀ����
�������ӷ���ʽ�У��������
| A����AlCl3��Һ�м��������ˮ��Al3+ + 3 NH3��H2O = Al(OH)3��+ 3NH4+ |
| B��������������Һ�м����������ữ�Ĺ���������Һ��2Fe2+ + H2O2 + 2H+ = 2Fe3+ +2H2O |
| C��̼���Ⱶ��Һ�м����������������Һ�� Ba2+ +2HCO3��+2OH��=BaCO3��+H2O + CO32- |
| D��ͭ�ܽ���ϡ�����У�Cu+4H�� +2NO3-=Cu2++ 2NO2 ��+2H2O |
D
ͭ�ܽ���ϡ����ķ���ʽ�� 3Cu+8HNO3=3Cu(NO3)2+2NO��+4H2O
���ӷ���ʽ ���� 3Cu+8H+2NO3-="3Cu2+" +4H2O+2NO��
��D����

��ϰ��ϵ�д�
 һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ
 2Al �� 3Cl2��
2Al �� 3Cl2�� NH3���� 2H2O
NH3���� 2H2O H2��+Cl2��+2OH-
H2��+Cl2��+2OH- 2SO3
2SO3 + 2HCO3- + 2OH- = MgCO3��+ CO32- + 2H2O
+ 2HCO3- + 2OH- = MgCO3��+ CO32- + 2H2O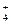 + OH��
+ OH��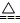 NH3��+H2O
NH3��+H2O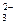 + H2O
+ H2O