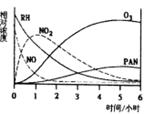
��Ŀ����
ʵ�����п�����ͼװ������ȡ����İ�����
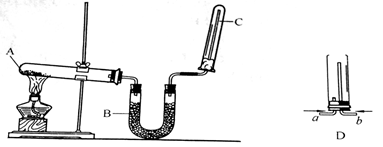
��1��д��A��������Ӧ�Ļ�ѧ����ʽ ��
��2��U�ι�B�����ŵ�ҩƷ�� ��
��3��ʵ������ȡ��������ʱ����ʵ������ȡ���������巢��װ����ͬ���� ��
A������ B������ C������ D��������̼
��4���Թ�C�Ĺܿڴ�Ҫ����һС�������������������� ��
��5��������ʾ�����������ڴ����е�ȼ����Ӧ���ɵ�����ˮ����Ϊ��֤����ʵ��ijͬѧ�����һ��ʵ�鷽����װ�ü�D����д���÷�Ӧ�Ļ�ѧ����ʽ ��

��1��д��A��������Ӧ�Ļ�ѧ����ʽ ��
��2��U�ι�B�����ŵ�ҩƷ�� ��
��3��ʵ������ȡ��������ʱ����ʵ������ȡ���������巢��װ����ͬ���� ��
A������ B������ C������ D��������̼
��4���Թ�C�Ĺܿڴ�Ҫ����һС�������������������� ��
��5��������ʾ�����������ڴ����е�ȼ����Ӧ���ɵ�����ˮ����Ϊ��֤����ʵ��ijͬѧ�����һ��ʵ�鷽����װ�ü�D����д���÷�Ӧ�Ļ�ѧ����ʽ ��
��1��2NH4Cl + Ca(OH)2CaCl2 + 2H2O + 2NH3�� ��2����ʯ��
��3��B ��4����ֹNH3�����������ʹ�ռ����������
��5��4NH3+3O2 2N2+6H2O
2N2+6H2O
��3��B ��4����ֹNH3�����������ʹ�ռ����������
��5��4NH3+3O2
 2N2+6H2O
2N2+6H2O�����������1��ʵ���Ҽ����Ȼ�粒�������ʯ�ҵĻ�����ư�������Ӧ����ʽΪ2NH4Cl + Ca(OH)2CaCl2 + 2H2O + 2NH3����
��2��BΪ����װ�ã����ﰱ���ü��Ը��������ʯ�һ�����������ƻ������ơ�
��3���ư����ķ���װ��Ϊ��������������ȡ���壬������������������غͶ������̵Ļ�������ȸ�����ع��壩װ����ͬ�����������ƶ�����̼���巢��װ����ͬ��������Һ�岻���������塣�������ķ���װ��Ϊ������Һ������������װ�á�
��4��������Է�������С���˶��ٶȿ죬C�ܿڷ�һ�����������Ƿ�ֹ�������������������ʹ�ռ������������
��5��������������ȼ�����ɵ�����ˮ�ķ���ʽΪ4NH3+3O2
 2N2+6H2O��
2N2+6H2O����������ס���������Ʊ��ķ���װ�á�

��ϰ��ϵ�д�
�����Ŀ