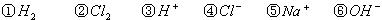��Ŀ����
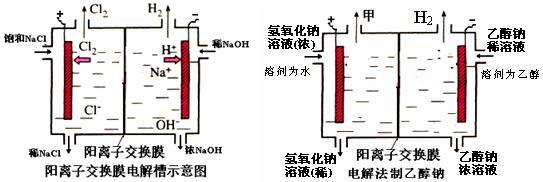
��ҵ���������������õ����е�ⱥ��ʳ��ˮ��Ϊ�˱��������֮�䷢����Ӧ�����������ӽ���Ĥ�����۸��������֣���ͼ����ͼ��Ϊ���۵�ʾ��ͼ��
��1�����������ӽ���Ĥ��ֻ������Һ�е�
��H2��Cl2��H+��Cl-��Na+��OH-
��2��д���ڵ����������������ĵ缫����ʽ��
��3����֪ij����ÿСʱ����10%������������Һ10kg��ÿСʱ���ռ�������µ�����896L���������ߵ�ˮ����������ͨ���������ϼ��㣬��������������������Һ����������Ϊ
��4��ij��ѧ������ȤС��������õ�ⷨ��ȡ�Ҵ��ƵĹ�ҵ���������õĵ�����ͼ��ʾ�����Ҫ�����õĽ���Ĥ�����÷�������ͨ�����ڵ����������ĵ�ԭ�����������ƺ��Ҵ����ش��������⣺
��д���ڵ����������������ĵ缫����ʽ
�������Ҵ��Ƶ��Ҵ���Һ�з���õ������Ҵ��ƹ���ķ����ǣ�
��1�����������ӽ���Ĥ��ֻ������Һ�е�
�ۢ�
�ۢ�
ͨ���������������ı�ţ���H2��Cl2��H+��Cl-��Na+��OH-
��2��д���ڵ����������������ĵ缫����ʽ��
2Cl��-2e-=Cl2��
2Cl��-2e-=Cl2��
����3����֪ij����ÿСʱ����10%������������Һ10kg��ÿСʱ���ռ�������µ�����896L���������ߵ�ˮ����������ͨ���������ϼ��㣬��������������������Һ����������Ϊ
35.7%
35.7%
����4��ij��ѧ������ȤС��������õ�ⷨ��ȡ�Ҵ��ƵĹ�ҵ���������õĵ�����ͼ��ʾ�����Ҫ�����õĽ���Ĥ�����÷�������ͨ�����ڵ����������ĵ�ԭ�����������ƺ��Ҵ����ش��������⣺

��д���ڵ����������������ĵ缫����ʽ
2CH3CH2OH+2e-=2CH3CH2O-+H2��
2CH3CH2OH+2e-=2CH3CH2O-+H2��
���������Ҵ��Ƶ��Ҵ���Һ�з���õ������Ҵ��ƹ���ķ����ǣ�
�����ᾧ
�����ᾧ
����������1�������ӽ���Ĥֻ����������ͨ�����������ӡ����Ӳ���ͨ����
��2�������к͵�Դ�������������ǵ��ص�����������ʧ���ӵ�������Ӧ��
��3����������������������ɵ��������Ƶ���������Һ���ٵ���������������Һ���ӵ��������Ƶ����������ٸ�������������ʽ���㼴�ɣ�
��4���ٵ����к͵�Դ�ĸ����������ǵ��ص������������õ��ӵĻ�ԭ��Ӧ��
�ڴ���Һ���������ʵķ����������ᾧ�������ʳ�ε������ᾧ��
��2�������к͵�Դ�������������ǵ��ص�����������ʧ���ӵ�������Ӧ��
��3����������������������ɵ��������Ƶ���������Һ���ٵ���������������Һ���ӵ��������Ƶ����������ٸ�������������ʽ���㼴�ɣ�
��4���ٵ����к͵�Դ�ĸ����������ǵ��ص������������õ��ӵĻ�ԭ��Ӧ��
�ڴ���Һ���������ʵķ����������ᾧ�������ʳ�ε������ᾧ��
����⣺��1�������ӽ���Ĥֻ���������������ӡ�������ͨ�����������Ӻͷ��Ӿ�����ͨ�����ʴ�Ϊ���ۢݣ�
��2�������к͵�Դ�������������ǵ��ص������������������ӷ���ʧ���ӵ�������Ӧ����2Cl--2e-=Cl2�����ʴ�Ϊ��2Cl--2e-=Cl2����
��3������������=
M=
��2g/mol=80g�����ˮʱ�������������������Ƶ����ʵ���֮��Ϊ1��2�����������������Ƶ�����=2
��40g/mol=3200g����Һ���ӵ�����Ϊ���ӵ���Ԫ�ص�������ȥ��������������=2��
��23g/mol-80g=1760g����Һ�������������Ƶ�����Ϊ10000g��10%+3200g=4200g����Һ������Ϊ10000g+1760g=11760g��
��������������������Һ����������=
��100%=35.7%��
�ʴ�Ϊ��35.7%��
��4���ٵ����к͵�Դ�ĸ����������ǵ��ص��������������Ҵ��õ��ӷ�����ԭ��Ӧ����2CH3CH2OH+2e-=2CH3CH2O-+H2����
�ʴ�Ϊ��2CH3H2OH+2e-=2CH3CH2O-+H2����
�ڴ���Һ���������ʵķ����������ᾧ���ʴ�Ϊ�������ᾧ��
��2�������к͵�Դ�������������ǵ��ص������������������ӷ���ʧ���ӵ�������Ӧ����2Cl--2e-=Cl2�����ʴ�Ϊ��2Cl--2e-=Cl2����
��3������������=
| V |
| Vm |
| 896L |
| 22.4L/mol |
| 896L |
| 22.4L/mol |
| 896L |
| 22.4L/mol |
��������������������Һ����������=
| 4200g |
| 11760g |
�ʴ�Ϊ��35.7%��
��4���ٵ����к͵�Դ�ĸ����������ǵ��ص��������������Ҵ��õ��ӷ�����ԭ��Ӧ����2CH3CH2OH+2e-=2CH3CH2O-+H2����
�ʴ�Ϊ��2CH3H2OH+2e-=2CH3CH2O-+H2����
�ڴ���Һ���������ʵķ����������ᾧ���ʴ�Ϊ�������ᾧ��
���������⿼���˵��ԭ����֪ʶ�㣬�ѶȲ����״����ǣ�3���⣬ע��������������Һʱ�����������������ӵ����ʵ���֮��Ϊ1��2����ȷ��Һ���������ļ��㷽����

��ϰ��ϵ�д�
 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
�����Ŀ