��Ŀ����
��16�֣���֪FeSO4�ڲ�ͬ�����·ֽ�õ��IJ��ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��ij�о�С��̽���ھƾ���Ƽ���������FeSO4�ֽ����������֪SO3���۵���16.8�棬�е���44.8�档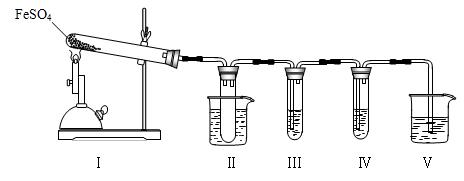
��1��װ�â���Թ��в�װ�κ��Լ�����������______________________
�Թܽ�����50���ˮԡ�У�Ŀ����______________________________
��2��װ�â��װ�â���������̽����ʵ���������ɷ֡������ʵ����ƣ���д�����Լ���Ԥ����������ۡ�
��ѡ�Լ���3 mol��L��1 H2SO4��6 mol��L��1 NaOH��0.5 mol��L��1 BaCl2��0.5 mol��L��1 Ba(NO3)2��
0.01 mol��L��1����KMnO4��Һ��0.01 mol��L��1��ˮ��
| �����Լ� | Ԥ������ͽ��� |
| װ�â���Թ��м���__________ ___�� | ����������ɫ������֤����������к���SO3�� |
| װ�â����Թ��м���_______ _________�� | ______________________________ ______________________________ ______________________________ ______________________________ |
��16�֣�
��1����ֹ��Һ������װ�â�2�֣� ��ֹSO3Һ�������̣�2�֣�
��2����10�֣�
�������Լ� Ԥ������ͽ��� װ�â���Թ���װ��BaCl2��Һ����3�֣� ����������ɫ������֤����������к���SO3�� װ�â����Թ���װ������KMnO4��Һ����3�֣� ����Һ�Ϻ�ɫ��ȥ��֤����������к���SO2����2�֣�
����Һ�Ϻ�ɫ�����Ա仯��֤����������в���SO2����2�֣�
��3��NaOH��Һ��2�֣������Լ� Ԥ������ͽ��� װ�â���Թ���װ��BaCl2��Һ����3�֣� ����������ɫ������֤����������к���SO3�� װ�â����Թ���װ����ˮ����3�֣� ����ˮ��ɫ��ȥ��֤����������к���SO2����2�֣�
����ˮ��ɫ�����Ա仯��֤����������в���SO2����2�֣�
����

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д���16�֣���֪FeSO4�ڲ�ͬ�����·ֽ�õ��IJ��ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��ij�о�С��̽���ھƾ���Ƽ���������FeSO4�ֽ����������֪SO3���۵���16.8�棬�е���44.8�档
��1��װ�â���Թ��в�װ�κ��Լ�����������______________________
�Թܽ�����50���ˮԡ�У�Ŀ����______________________________
��2��װ�â��װ�â���������̽����ʵ���������ɷ֡������ʵ����ƣ���д�����Լ���Ԥ����������ۡ�
��ѡ�Լ���3 mol��L��1 H2SO4��6 mol��L��1 NaOH��0.5 mol��L��1 BaCl2��0.5 mol��L��1 Ba(NO3)2��
0.01 mol��L��1����KMnO4��Һ��0.01 mol��L��1��ˮ��
| �����Լ� | Ԥ������ͽ��� |
| װ�â���Թ��м���__________ ___�� | ����������ɫ������֤����������к���SO3�� |
| װ�â����Թ��м���_______ _________�� | ______________________________ ______________________________ ______________________________ ______________________________ |
��3��װ�â��������Ƿ�ֹβ����Ⱦ�������ձ���Ӧ������Լ��� ��
��9�֣�����֪FeSO4�ڲ�ͬ�����·ֽ�õ��IJ��ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��ij�о�С��̽���ھƾ���Ƽ���������FeSO4�ֽ����������֪SO3���۵���16.8�棬�е���44.8��
��װ�â���Թ��в�װ�κ��Լ����������� ���Թܽ�����50���ˮԡ�У�Ŀ���� ��
| �����Լ� | Ԥ����������� |
| װ�â���Թ��м��� �� | ����������ɫ������֤����������к���SO3 |
| װ�â����Թ��м��� �� |  |
 ����������̽����ʵ���������ɷ֡������ʵ����ƣ���д�����Լ���Ԥ����������ۡ���ѡ�Լ���3mol��L��1H2SO4��6 mol��L��1NaOH��0.5 mol��L��1BaCl2��0.5 mol��
����������̽����ʵ���������ɷ֡������ʵ����ƣ���д�����Լ���Ԥ����������ۡ���ѡ�Լ���3mol��L��1H2SO4��6 mol��L��1NaOH��0.5 mol��L��1BaCl2��0.5 mol�� L��1Ba(NO3)2��0.01 mol��L��1����KMnO4��Һ��0.01 mol��L��1��ˮ
L��1Ba(NO3)2��0.01 mol��L��1����KMnO4��Һ��0.01 mol��L��1��ˮ��װ�â��������Ƿ�ֹβ����Ⱦ�������ձ���Ӧ������Լ��� ��
��֪FeSO4�ڲ�ͬ�����·ֽ�õ��IJ��ﲻͬ��������FeO��SO3��Ҳ������Fe2O3��SO3��SO2��ij�о�С��̽���ھƾ���Ƽ���������FeSO4�ֽ����������֪SO3���۵���16.8�棬�е���44.8�档

��1��װ�â���Թ��в�װ�κ��Լ�����������_______________________���Թܽ�����50���ˮԡ�У�Ŀ����________________________________��
��2��װ�â��װ�â���������̽����ʵ���������ɷ֡������ʵ����ƣ���д�����Լ���Ԥ����������ۡ�
��ѡ�Լ���3 mol��L��1 H2SO4��6 mol��L��1 NaOH��0.5 mol��L��1 BaCl2��0.5 mol��L��1 Ba(NO3)2��0.01 mol��L��1����KMnO4��Һ��0.01 mol��L��1��ˮ��
|
�����Լ� |
Ԥ������ͽ��� |
|
װ�â���Թ��м���________________�� |
����������ɫ������֤����������к���SO3�� |
|
װ�â����Թ��м���________________�� |
______________________________ ______________________________ ______________________________ ______________________________ |
��3��װ�â��������Ƿ�ֹβ����Ⱦ�������ձ���Ӧ������Լ��� ��

