��Ŀ����
ij��ѧС���Ա�����Ϊԭ����ȡ����������������Ƶõı�����������м���ˮ���ʵ�顣
I����ȡ�������������֪�й����ʵķе����±���
|
���� |
�״� |
������ |
��������� |
|
�е�/�� |
64.7 |
249 |
199.6 |
ʵ��װ�����£�

��1��������һ�����Һ��ʱ��������Ũ�����������
��
����Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ��
��
��2����Һʱ�ϲ�Һ��� �������������ʱ�¶ȿ����� ��
��3������ȡ���̱Ƚ̲��������������Ʊ����Ӻܶ࣬д���ô˹����Ʊ�����������������ŵ㣺 �� ��
II���������������ˮ��
��4��д���������������ˮ��Ļ�ѧ��Ӧ����ʽ ��
��5��д���������������ˮ��ʵ��װ����A������ ��
III������������ж���ͬ���칹��
��6���������������Ľṹ
�ٿ��Է���������Ӧ�����ڷ����廯����۲��߱�������״�ṹ�ܲ�����FeCl3������ɫ��Ӧ������ �֡�
��16�֣���1��Ũ�����ܶȽϴ����뱽���ᡢ�״���Ϸų������������״��ӷ���(2��)
C6H5CO18OH+CH3OH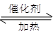 C6H5COOCH3+H218O
��(2��)
C6H5COOCH3+H218O
��(2��)
��2����Һ©�����Ͽڣ�199.6�����ҡ�(��2��)��3�����ʸߣ����ȸߡ�(2��)
��4��C6H5COOCH3 + NaOH �� C6H5COONa + CH3OH��(2��)
��5������������(2��) ��6��12 (2��)
��������

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д����� | �״� | ������ | ��������� |
�е�/�� | 64.7 | 249 | 199.6 |
��.�ϳɱ���������ֲ�Ʒ
��Բ����ƿ�м���
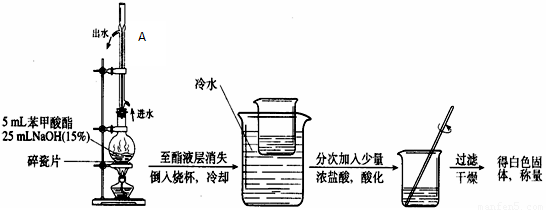
��1��Ũ�����������________________________��
����Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ_____________��
��2��������λͬѧ�ֱ����������ͼ����ʵ���Һϳɱ����������װ�ã��г������ͼ�������������ȥ����

�����л���ķе㣬��ò���_____________װ�ã���ס����ҡ�����
������_______________________________________��
��3����Ӧ��CH3OHӦ������������_______________________________________��
��.�ֲ�Ʒ�ľ���
��4������������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ��������������̽��о��ƣ���������ͼ�з�����������ǡ���������������ơ�

��5�����������м���Na2CO3��Һ�����Һ©���������ã�Ҫ�õ��Ͳ㣬����������____________________________________________________________________��
��6��ͨ�����㣬����������IJ���Ϊ__________________________��
ij��ѧС���Ա�����Ϊԭ����ȡ����������������Ƶõı�����������м���ˮ���ʵ�顣
I����ȡ�������������֪�й����ʵķе����±���
| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |
ʵ��װ�����£�
��1��������һ�����Һ��ʱ��������Ũ�����������
��
����Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ��
��
��2����Һʱ�ϲ�Һ��� �������������ʱ�¶ȿ����� ��
��3������ȡ���̱Ƚ̲��������������Ʊ����Ӻܶ࣬д���ô˹����Ʊ�����������������ŵ㣺 �� ��
II���������������ˮ��
��4��д���������������ˮ��Ļ�ѧ��Ӧ����ʽ ��
��5��д���������������ˮ��ʵ��װ����A������ ��
III������������ж���ͬ���칹��
��6���������������Ľṹ
�ٿ��Է���������Ӧ�����ڷ����廯����۲��߱�������״�ṹ�ܲ�����FeCl3������ɫ��Ӧ������ �֡�
(14��)ij��ѧС���Ա�����Ϊԭ�ϣ���ȡ�������������֪�й����ʵķе����±���
| ���� | �״� | ������ | ��������� |
| �е㣯�� | 64.7 | 249 | 199.6 |
������ƿ�м���12.2g �������20 mL �״����ܶ�Լ0.79g �� mL��1) ����С�ļ���3 mL Ũ���ᣬ���Ⱥ�Ͷ�˼������Ƭ��С�ļ���ʹ��Ӧ��ȫ���ñ���������ֲ�Ʒ��
(1)Ũ�����������_________������Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ��__________________��
(2)������λͬѧ�ֱ����������ͼ����ʵ���Һϳɱ����������װ�ã��г������ͼ�������������ȥ���������л���ķе㣬��ò���__________װ�ã���ס����ҡ�����������___________________��

(3)��Ӧ��CH3 OH Ӧ������������__________________________________��
II���ֲ�Ʒ�ľ���
(4)����������ֲ�Ʒ���������������״������ᡢ�������ˮ�ȣ���������������ͼ���о��ƣ���������ͼ�з�����������ǡ���������������ơ�

(5)��������ͼ�м���Na2CO3��Һ���˷�Һ©���������ã�Ҫ�õ��л��㣬����������______________________________________________________________________
(6)ͨ�����㣬����
 ������IJ���Ϊ_________________________��
������IJ���Ϊ_________________________�� ij��ѧС���Ա�����Ϊԭ����ȡ����������������Ƶõı�����������м���ˮ���ʵ�顣
I����ȡ�������������֪�й����ʵķе����±���
| ���� | �״� | ������ | ��������� |
| �е�/�� | 64.7 | 249 | 199.6 |

��1��������һ�����Һ��ʱ��������Ũ�����������
��
����Ӧ����ˮ��������ͬλ��18O��д���ܱ�ʾ��Ӧǰ��18Oλ�õĻ�ѧ����ʽ��
��
��2����Һʱ�ϲ�Һ��� �������������ʱ�¶ȿ����� ��
��3������ȡ���̱Ƚ̲��������������Ʊ����Ӻܶ࣬д���ô˹����Ʊ�����������������ŵ㣺 �� ��
II���������������ˮ��


��4��д���������������ˮ��Ļ�ѧ��Ӧ����ʽ ��
��5��д���������������ˮ��ʵ��װ����A������ ��
III������������ж���ͬ���칹��
��6���������������Ľṹ
�ٿ��Է���������Ӧ�����ڷ����廯����۲��߱�������״�ṹ�ܲ�����FeCl3������ɫ��Ӧ������ �֡�