��Ŀ����
��A��B��C��D��E���ֶ�����Ԫ���У�A��B��C����Ԫ�ص�ԭ��������������A��C������B��ԭ�Ӹ�����Ϊ1��1��2��1�γɻ����������ʱA��B�γɵĻ������Һ̬��C��B�γɵĻ�����ʹ�̬��D��ԭ��������������࣬E�ĺ˵�������D��E���γ���̬����ED4��
(1)����D��ԭ�ӽṹʾ��ͼ�� ������Ԫ��ԭ�Ӱ뾶�Ӵ�С��˳���� (��Ԫ�ط���)��
(2)A��B�γɵĻ������У����Ǽ��Լ��Ļ�����ĽṹʽΪ ��
(3)C��B��ԭ�Ӹ�����Ϊ1��1�γɻ�����ĵ���ʽ�� ��
(4)D��E���γ��⻯����ȶ�����ǿ������˳���� (�����Ļ�ѧʽ)��
(5)�ɶ�����Ԫ����ɵ�ijЩ������SO2��O3��NO2-���ɻ���Ϊ�ȵ����壬����B��Dͬ���ڵ�Ԫ����ɵ����У�����N3-����Ϊ�ȵ���������� (�����Ҫ��������������)��
(6)д����ҵ����E���ʵĻ�ѧ����ʽ�� ��
(1)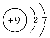 ��Na��Si��O��F��H
��Na��Si��O��F��H
(2)H��O��O��H
(3)
(4)HF��SiH4 (5)������̼��һ������������������
(6)2C��SiO2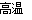 Si��2CO��
Si��2CO��
����������A��B��ԭ�Ӹ�����1��1��2��1�γ�Һ̬�������֪��AΪ�⣬BΪ����C��B��ԭ�Ӹ�����1��1��2��1�γɹ�̬�����CΪ�ƣ�D��E���γ���̬����ED4��D������������࣬��D�ڵ���A�壬E�ĺ˵�������EΪ�裬DΪ����