��Ŀ����
����ʵ���ͼ���Ľ��ۻ������ȷ���ǣ� ��| ʵ���ͼ�� | ���ۻ���� | |
| A | �����ھƾ��ƻ����ϼ����ۻ��������� | �����������ɵ���������и��۵� |
| B | ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ | c��CH3COO-��һ������c��Na+����c��NH4+��֮�� |
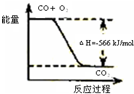
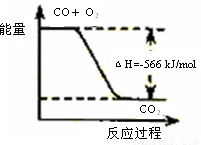
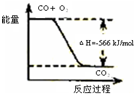
| C | �÷�Ӧ�Ȼ�ѧ����ʽΪ2CO��g��+O2��g��=2CO2��g����H=-566kJ/mol |  |
| D | ��������������ʢװ���Ũ���� | ����Ũ�����Ӧ |
A��A
B��B
C��C
D��D
���𰸡�������A�����������۵�������۵�ߣ������ھƾ��ƻ����ϼ����ۻ��������䣻
B��CH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ����Һ���ܳ����ԡ����Ի����ԣ���ϵ���غ��жϣ�
C���Ȼ�ѧ��Ӧ����ʽ�з�Ӧ������ʵ����뷴Ӧ�ų������������ȣ�
D������Ũ�����з����ۻ���
����⣺A�����������۵�������۵�ߣ������ھƾ��ƻ����ϼ����ۻ��������䣬���۽�����ȷ����A��ȷ��
B����Һ�ʵ����ԣ�����c��Na+��+c��NH4+��+c��H+��=c��CH3COO-��+c��OH-��������Һ�����ԣ���c��H+��=c��OH-������c��Na+��+c��NH4+��=c��CH3COO-��������Һ�����ԣ���c��H+����c��OH-������c��Na+��+c��NH4+����c��CH3COO-��������Һ�ʼ��ԣ���c��H+����c��OH-������c��Na+��+c��NH4+����c��CH3COO-������B����
C���Ȼ�ѧ��Ӧ����ʽ��ʾ2molCOȼ�շų�������Ϊ566kJ��ͼ��Ӧ��ʾ1molCOȼ�����ɶ�����̼�ų���������ϵ����Ӧ��ӦΪ-283kJ/mol����C����
D������Ũ���ᷴӦ����һ�����ܵ������ﱣ��Ĥ��ֹ�ڲ�������Ũ���������Ӧ���������ۻ�����D����
��ѡA��
���������⿼���������������ʡ�����Ũ�ȱȽϡ��Ȼ�ѧ��Ӧ����ʽ��Ũ�������ʵȣ��Ѷ��еȣ�ע��Bѡ����Һ������ԣ�Cѡ���������ľ���һ���Ȼ�ѧ����ʽע�⡰������˵�������ʡ��������������Ƿ�Ӧ���������������Ӧ��ϵ���������Dzμӷ�Ӧ�����ʵ�����
B��CH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ����Һ���ܳ����ԡ����Ի����ԣ���ϵ���غ��жϣ�
C���Ȼ�ѧ��Ӧ����ʽ�з�Ӧ������ʵ����뷴Ӧ�ų������������ȣ�
D������Ũ�����з����ۻ���
����⣺A�����������۵�������۵�ߣ������ھƾ��ƻ����ϼ����ۻ��������䣬���۽�����ȷ����A��ȷ��
B����Һ�ʵ����ԣ�����c��Na+��+c��NH4+��+c��H+��=c��CH3COO-��+c��OH-��������Һ�����ԣ���c��H+��=c��OH-������c��Na+��+c��NH4+��=c��CH3COO-��������Һ�����ԣ���c��H+����c��OH-������c��Na+��+c��NH4+����c��CH3COO-��������Һ�ʼ��ԣ���c��H+����c��OH-������c��Na+��+c��NH4+����c��CH3COO-������B����
C���Ȼ�ѧ��Ӧ����ʽ��ʾ2molCOȼ�շų�������Ϊ566kJ��ͼ��Ӧ��ʾ1molCOȼ�����ɶ�����̼�ų���������ϵ����Ӧ��ӦΪ-283kJ/mol����C����
D������Ũ���ᷴӦ����һ�����ܵ������ﱣ��Ĥ��ֹ�ڲ�������Ũ���������Ӧ���������ۻ�����D����
��ѡA��
���������⿼���������������ʡ�����Ũ�ȱȽϡ��Ȼ�ѧ��Ӧ����ʽ��Ũ�������ʵȣ��Ѷ��еȣ�ע��Bѡ����Һ������ԣ�Cѡ���������ľ���һ���Ȼ�ѧ����ʽע�⡰������˵�������ʡ��������������Ƿ�Ӧ���������������Ӧ��ϵ���������Dzμӷ�Ӧ�����ʵ�����

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
��2011?������һģ������ʵ���ͼ���Ľ��ۻ������ȷ���ǣ�������
|
����ʵ���ͼ���Ľ��ۻ������ȷ����
|
|
ʵ���ͼ�� |
���ۻ���� |
|
A |
�����ھƾ��ƻ����ϼ����ۻ��������� |
�����������ɵ���������и��۵� |
|
B |
ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ |
c(CH3COO-)һ������c(Na+)��c(NH4+)֮�� |
|
C |
|
�÷�Ӧ�Ȼ�ѧ����ʽΪ2CO(g)+O2(g)=2CO2(g)��H=-566 kJ/mol |
|
D |
��������������ʢװ���Ũ���� |
����Ũ�����Ӧ |
����ʵ���ͼ���Ľ��ۻ������ȷ����
|
| ʵ���ͼ�� | ���ۻ���� |
| A | �����ھƾ��ƻ����ϼ����ۻ��������� | �����������ɵ���������и��۵� |
| B | ijCH3COOH��CH3COONa��ɵ�������Һ�У�����һ������ˮ | c(CH3COO-)һ������c(Na+)��c(NH4+)֮�� |
| C | | �÷�Ӧ�Ȼ�ѧ����ʽΪ2CO(g)+O2(g)=2CO2(g)��H=-566 kJ/mol |
| D | ��������������ʢװ���Ũ���� | ����Ũ�����Ӧ |