��Ŀ����
�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ(C3H6)��
(1)��������ɵñ�ϩ��
��֪��C3H8(g)![]() CH4(g)+HC��CH(g)+H2(g) ��H1=156.6 kJ��mol-1
CH4(g)+HC��CH(g)+H2(g) ��H1=156.6 kJ��mol-1
CH3CH=CH2(g)![]() CH4(g)+HC��CH(g) ��H2=32.4 kJ��mol-1
CH4(g)+HC��CH(g) ��H2=32.4 kJ��mol-1
����ͬ�����£���ӦC3H3(g)![]() CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1
CH3CH=CH2(g)+H2(g)�ġ�H=_____kJ��mol-1
(2)�Ա���Ϊȼ����������ȼ�ϵ�أ���ص�����ͨ��O2��CO2������ͨ����飬�����������̼���Ρ���ط�Ӧ����ʽΪ___________���ŵ�ʱ��![]() �����ص�_______(���������)����
�����ص�_______(���������)����
(3)̼�⻯������ȫȼ������CO2��H2O�����³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH��5.60��c(H2CO3)��1.5��10-5 mol��L-1��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3![]()
![]() +H+��ƽ�ⳣ��K1��_____________������֪��10-5.60��2.5��10-6��
+H+��ƽ�ⳣ��K1��_____________������֪��10-5.60��2.5��10-6��
(4)�����£�0.1 mol��L-1NaHCO3��Һ��pH����8������Һc(H2CO3)______c(![]() )(�����������������)��ԭ����__________�������ӷ���ʽ�ͱ�Ҫ������˵������
)(�����������������)��ԭ����__________�������ӷ���ʽ�ͱ�Ҫ������˵������
(1)124.2
(2)C3H8+5O2![]() 3CO2+4H2O ��
3CO2+4H2O ��
(3)4.2��10-7 mol��L-1
(4)�� ![]() +H2O
+H2O![]()
![]() +
+![]() (��
(�� ![]()
![]()
![]() +H+)
+H+)
![]() +H2O
+H2O![]() H2CO3 +OH- ��
H2CO3 +OH- ��![]() ��ˮ��̶ȴ��ڵ���̶�
��ˮ��̶ȴ��ڵ���̶�
��������1����-�ڵ�C3H8��g��![]() CH3CH=CH2��g��+H2��g�� ��H=��H1-��H2=124.2 kJ��mol-1��
CH3CH=CH2��g��+H2��g�� ��H=��H1-��H2=124.2 kJ��mol-1��
��2���õ���У�O2�������õ��ӣ���CO2��ϳ�![]() �����ɵ�
�����ɵ�![]() ���ƶ���
���ƶ���
��3��K1=![]() =4.2��10-7 mol��L-1��
=4.2��10-7 mol��L-1��
��4��pH��8����Һ�Լ��ԣ���˵��ˮ��̶ȴ��ڵ���̶ȣ�ˮ�⣺![]() +H2O
+H2O![]() H2CO3 +OH-�����룺
H2CO3 +OH-�����룺![]() +H2O
+H2O![]()
![]() +H3O+������c��H2CO3����c��
+H3O+������c��H2CO3����c��![]() ����
����

 HCO3-+H+��ƽ�ⳣ��K1=
HCO3-+H+��ƽ�ⳣ��K1= CO32-+H+��HCO3-+H2O
CO32-+H+��HCO3-+H2O H2CO3+OH-��
H2CO3+OH-��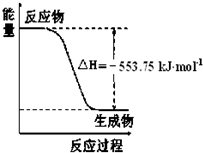 ��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���
��1����˹������Ϊ�����ܻ�ѧ������һ����ɻ��������ɣ��������̵���