��Ŀ����
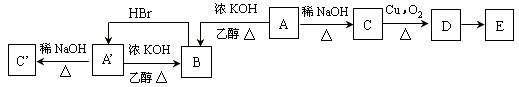
(��С��15��) A��B��C��D��Ԫ�صĺ˵�����������ӣ����ǵ����ӵĵ��Ӳ�����ͬ��������������Ϊ8��Aԭ�ӵ�L���������K��M�������֮����ȣ�Dԭ�ӵ�K��L�������֮�͵��ڵ���������һ�롣�ش��������⣺
(1)��Ԫ�صķ���������A______��B______��C________��D______��
(2)д��B��DԪ�ص����ӽṹʾ��ͼ������B��______________________�� D��______________________��
(3)�õ���ʽ��ʾA��CԪ���γɵ����ӻ�����Ĺ��̣� ________________________________��
(4)��A��B��C��D���������ˮ�����У�
�Ƚ������������ǿ����____________________________��
�Ƚ����м�ļ���ǿ����____________________________��
(1) S��Cl��K��Ca��4�֣�ÿ��1�֣�
(2) Cl��![]() ��2�֣� Ca2+
��2�֣� Ca2+![]() ��2�֣�
��2�֣�
(3)![]() ��3�֣�
��3�֣�
(4) ���ԣ�HClO4��H2SO4 ��2�֣� ���ԣ�KOH��Ca(OH)2��2�֣�

(��С��15��)Ϊ�ⶨ������CO2������������ʵ�顣
| ��0.1mol/L�ı������0.01mol/L�ı����� | �� | ��0.1mol/L�ı�����ζ�δ֪Ba(OH)2��Һ10mL��ȥ����19.60 mL | �� | ��Ba(OH)2��Һ���տ����е�CO2 | �� | �� �� | �� | ȡ��Һ20mL����0.01mol/L������ζ���ȥ����34.8mL |
��1��Ϊ���ñ���Һ����ѡȡ�����һ������ ��
��������ƽ ������ƿ �۵ζ��� ����Ͳ ���ձ� ��ͷ�ι� �߲�����
A���٢ڢݢ� B���ڢܢݢޢ� C���ڢ٢ޢ� D���ڢܢݢ�
��2���ζ�����ʱ������ ���۾�ע�� ��
��3��ȡ����Ba(OH)2��Һ10mL����100mL����ƿ�У���ˮϡ�����̶ȣ���ϡ�ͺ����Һ�����ܱ�������������10L�����������ˡ�����˵�ԭ���� ��
��4����ʵ���������������CO2���������Ϊ ��
��5����ʵ���У�����һ�εζ�ʱʹ�õ���ʽ�ζ���δ����������������Һ�����еڶ��εζ�������ʵ������ֵ����ƫ�ߡ�ƫ�ͻ���Ӱ�죩 ��