��Ŀ����
��������A�к��н���LiԪ�أ���ˮǿ��ˮ��,��Ҫ�����л��ϳɺ�ҩ�����죬�Ǿ�������ǰ���Ĵ�����ϡ���һ�������£�2.30g����A��5.35gNH4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L����C (��״��)����֪����C��������ˮ�õ�������Һ, �����ˮB�����ɽ�������D��������
�ش��������⣺
��1��A�Ļ�ѧʽ��????? �� C�ĵ���ʽ��????? ��
��2��д��������A�����ᷴӦ�Ļ�ѧ����ʽ��????? ��
��3��ijͬѧͨ���������ϵ�֪����A�����ʣ�
������ҵ�Ͽ��ý���D��Һ̬��C�����������·�Ӧ���Ʊ�A���ʣ�������A����Ϊ��ɫ���壬���ƵõĴ�Ʒ�����ǻ�ɫ�ġ�
��������A�۵�390�����е�430�����ܶȴ��ڱ���ױ�,������ú�ͣ���ˮ��Ӧ���ң�ҲҪ����Ӵ���;ƾ����ڿ����л�����A�����ֽ⣬�����ǿ�������ҷֽ⣬�����ᱬը.��750��800���ֽ�Ϊ������E������C��
������A��750��800���ֽ�ķ���ʽΪ��????? ��
�����õ�����A���ֱܴ��ʶ�����ʹ�ã��轫�����١�����������������ñ���ױ����串�ǣ�Ȼ���������ñ���ױ�ϡ������ˮ�Ҵ����Խ����仯ѧԭ��????? ��
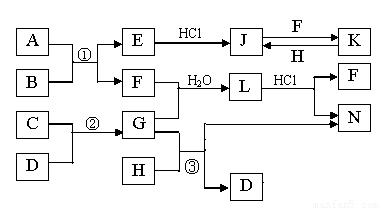
��4����ҵ�Ʊ�����D������ͼ���£�

���������в������ƣ�????? ��
������ƽ��ԭ�����Ͳ������м�ѹ��Ŀ�ģ�????? ��
��1��LiNH2??  ???
???
��2��LiNH2 +2HCl =LiCl + NH4Cl??
��3���� 3LiNH2  Li3N + 2NH3��
Li3N + 2NH3��
�� LiNH2�ܶȴ��ڱ���ױ��Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ����Ը�LiNH2��Ӧ������ʽΪ��LiNH2+C2H5OH C2H5 OLi + NH3�ɽ������١�
C2H5 OLi + NH3�ɽ������١�
��4��������Ũ������ȴ�ᾧ
�� LiCl�qH2O(S)  LiCl (s) + H2O(g)? ��Сѹǿ������������ƽ�����������ƶ�����������ˮLiCl���Ʊ���
LiCl (s) + H2O(g)? ��Сѹǿ������������ƽ�����������ƶ�����������ˮLiCl���Ʊ���
��������
�����������1�����������Ϣ������֪��������A�к���LiԪ�أ���ˮǿ��ˮ����������һ�������£�2.30g����A��5.35gNH4Cl����ǡ����ȫ��Ӧ�����ɹ���B��4.48L����C (��״��)����֪����C��������ˮ�õ�������Һ, �����ˮB�����ɽ�������D��������������֪CΪNH3�������ݵ�����ڵĽ����Ȼ�����Եõ��������ʺ�������֪ʶ������֪BΪLiCl�������A��NH4Cl���巴Ӧ�ɱ�Ϊ��A+ NH4Cl �� LiCl + NH3��NH4Cl��Ħ������Ϊ53.5g/mol��5.35gNH4ClΪ0.1mol����Ӧ���ɵ�LiClҲӦΪ0.1mol����ô������A�к�LiԪ��ҲΪ0.1mol���ٸ��������غ��ԭ���غ㣨ԭ�ӵ��������Ŀ��Ӧǰ����ͬ�����Ϳ��Ƴ��÷�Ӧ�Ļ�ѧ����ʽΪӦ��LiNH2 + NH4Cl = LiCl + NH3���ɴ˿�֪A��LiNH2��LiNH2��Ħ������Ϊ23.0g/mol��2.30g LiNH2Ϊ0.1mol�����ϻ�ѧ��Ӧ����ʽ�еĻ�ѧ�������� NH3���ӵĵ���ʽΪ��
��2�� ���ݻ�����A��LiNH2����ˮǿ��ˮ�⣬������LiOH��NH3 �������������ᷴӦ��ӦΪ��LiNH2 +2HCl =LiCl + NH4Cl��
��3�������������غ㣨ԭ���غ㣩�������Ƴ�LiNH2��750��800���ֽ�ķ���ʽΪ��3LiNH2 Li3N + 2NH3����
Li3N + 2NH3����
���������LiNH2�й����ʣ��ñ���ױ����串�ǣ�Ȼ���������ñ���ױ�ϡ������ˮ�Ҵ����ٱ��ʵ�LiNH2�Ļ�ѧԭ������LiNH2�ܶȴ��ڱ���ױ����Ҳ��������ǣ����Կ��ñ���ױ����и��ǣ��Ҵ����Ը�LiNH2��Ӧ��LiNH2+C2H5OH C2H5OLi + NH3���ɽ������١����������ע�⣬Ӧ�ش���ǽ����仯ѧԭ���������ǽ�����ԭ��
C2H5OLi + NH3���ɽ������١����������ע�⣬Ӧ�ش���ǽ����仯ѧԭ���������ǽ�����ԭ��
��4��ͨ����ҵ�Ʊ�����Li������ͼ��֪������������Ҫ��LiCl��Һ���LiCl�qH2O���壬���ԣ������ӦΪ������Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ��������еIJ��������й��ˡ�ϴ�ӣ���Ӧ��������Ũ������ȴ�ᾧ����
����������Ҫ��LiCl�qH2O�����ڼ�ѹ��������Ȼ��������м��ȣ�200����������ˮLiCl��Ȼ������������ڵ�LiCl�Ƶý���Li����˸���LiCl�qH2O������ȥ�ᾧˮ������ˮLiCl�ķ�Ӧ��LiCl�qH2O(S)  LiCl (s) + H2O(g)����һ��������������ķ�Ӧ�����Լ�Сѹǿ��������ƽ��LiCl�qH2O(S)
LiCl (s) + H2O(g)����һ��������������ķ�Ӧ�����Լ�Сѹǿ��������ƽ��LiCl�qH2O(S)  LiCl (s) + H2O(g)���������ƶ�����������ˮLiCl���Ʊ���
LiCl (s) + H2O(g)���������ƶ�����������ˮLiCl���Ʊ���
���㣺��ѧ�������̣�Ԫ�ؼ��仯��������

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�