��Ŀ����
������������ȷ���ǣ� ��
| A��25��ʱ����Mg��OH��2������Һ�м���������NH4Cl���壬c��Mg2+������ |
| B��һ���¶��£�IL 1mol��L�İ�ˮ��2L 0��5mol��L�İ�ˮ�У�n��NH4+��ǰ�߶� |
| C����ͬ�������ͬ���ʵ���Ũ�ȵ����ᡢ���ᣬϡ����ͬ��������Һ��pH������<���� |
| D��0��2mol��L��һԪ��HX��0��1mol��L��KOH��Һ��������������Һ�У�һ���У�a��H+��+c��K+��=c��OH-��+c��X-�� |
B
��

��ϰ��ϵ�д�
�����Ŀ

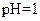 ����Һ�У�
����Һ�У�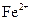 ��
��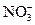 ��
��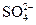 ��
��
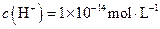 ����Һ�У�
����Һ�У�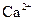 ��
�� ��
��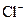 ��
��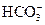
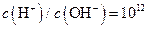 ����Һ�У�
����Һ�У�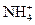 ��
��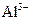 ��
�� ����Һ�У�
����Һ�У�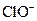 ��
��