��Ŀ����
��2013?������һģ����Ҫ�ľ�ϸ��ѧƷM��N����������������Ϳ�ϡ�ɱ����ȣ��ϳ�·����ͼ��ʾ��

��֪��iii N�Ľṹ��ʽ��

��ش��������⣺
��1��A���������������
��2��X�Ľṹ��ʽ��
��3��C�ͼ״���Ӧ�IJ�����Ծۺ��γ��л��������þۺϷ�Ӧ�Ļ�ѧ����ʽ��
 ��
��
��4��E�Ľṹ��ʽ��
 ��
��
��5������˵����ȷ����
a��E�ܷ�����ȥ��Ӧ
b��1molM���4mol����
c��X��Y��ͬϵ��
d��G������˳���칹��
��6����Y����D�Ļ�ѧ����ʽ��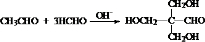
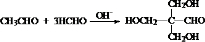 ��
��
��7��Z�Ľṹ��ʽ��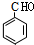
 ��
��

��֪��iii N�Ľṹ��ʽ��

��ش��������⣺
��1��A���������������
�ǻ���ȩ��
�ǻ���ȩ��
����A����B�ķ�Ӧ��������ȥ��Ӧ
��ȥ��Ӧ
����2��X�Ľṹ��ʽ��
CH3CH2CHO
CH3CH2CHO
����3��C�ͼ״���Ӧ�IJ�����Ծۺ��γ��л��������þۺϷ�Ӧ�Ļ�ѧ����ʽ��


��4��E�Ľṹ��ʽ��


��5������˵����ȷ����
bc
bc
��a��E�ܷ�����ȥ��Ӧ
b��1molM���4mol����
c��X��Y��ͬϵ��
d��G������˳���칹��
��6����Y����D�Ļ�ѧ����ʽ��
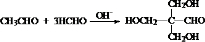
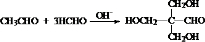
��7��Z�Ľṹ��ʽ��


�������л���X��HCHO������Ϣi�ķ�Ӧ�����A�ķ���ʽΪC4H8O2��֪��XΪCH3CH2CHO��AΪHOCH2CH��CH3��CHO��A������ȥ��Ӧ��ȥ1����H2O����B����BΪCH2�TC��CH3��CHO��B��������CΪCH2�TC��CH3��COOH��
����Ϣiii��N�Ľṹ�������Ϣii�з�Ӧ��֪���γ�M������Ϊ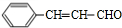 ��
�� ��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ
��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ ����GΪ
����GΪ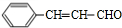 ��C��Eͨ��������Ӧ����M��
��C��Eͨ��������Ӧ����M��
��Y��E��ת�������E�Ľṹ��֪��Y�����������ٽ��G�Ľṹ��֪��������Z�к��б�������Ϸ�Ӧ��Ϣi��֪��YΪCH3CHO��ZΪ ����FΪ
����FΪ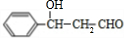 ��
��
��E�Ľṹ��֪��1����CH3CHO��3����HCHO������Ϣi��Ӧ����D��DΪ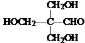 ��D�����������ӳɷ�Ӧ����E��
��D�����������ӳɷ�Ӧ����E�� �����ݴ˽��
�����ݴ˽��
����Ϣiii��N�Ľṹ�������Ϣii�з�Ӧ��֪���γ�M������Ϊ
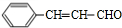 ��
�� ��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ
��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ ����GΪ
����GΪ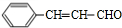 ��C��Eͨ��������Ӧ����M��
��C��Eͨ��������Ӧ����M����Y��E��ת�������E�Ľṹ��֪��Y�����������ٽ��G�Ľṹ��֪��������Z�к��б�������Ϸ�Ӧ��Ϣi��֪��YΪCH3CHO��ZΪ
 ����FΪ
����FΪ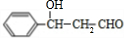 ��
����E�Ľṹ��֪��1����CH3CHO��3����HCHO������Ϣi��Ӧ����D��DΪ
 ��D�����������ӳɷ�Ӧ����E��
��D�����������ӳɷ�Ӧ����E�� �����ݴ˽��
�����ݴ˽������⣺�л���X��HCHO������Ϣi�ķ�Ӧ�����A�ķ���ʽΪC4H8O2��֪��XΪCH3CH2CHO��AΪHOCH2CH��CH3��CHO��A������ȥ��Ӧ��ȥ1����H2O����B����BΪCH2�TC��CH3��CHO��B��������CΪCH2�TC��CH3��COOH��
����Ϣiii��N�Ľṹ�������Ϣii�з�Ӧ��֪���γ�M������Ϊ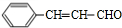 ��
�� ��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ
��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ ����GΪ
����GΪ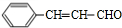 ��C��Eͨ��������Ӧ����M��
��C��Eͨ��������Ӧ����M��
��Y��E��ת�������E�Ľṹ��֪��Y�����������ٽ��G�Ľṹ��֪��������Z�к��б�������Ϸ�Ӧ��Ϣi��֪��YΪCH3CHO��ZΪ ����FΪ
����FΪ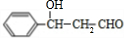 ��
��
��E�Ľṹ��֪��1����CH3CHO��3����HCHO������Ϣi��Ӧ����D��DΪ ��D�����������ӳɷ�Ӧ����E��
��D�����������ӳɷ�Ӧ����E�� ����
����
��1��������������֪��AΪHOCH2CH��CH3��CHO�������ǻ���ȩ����A��B��HOCH2CH��CH3��CHO������ȥ��Ӧ��ȥ1����H2O����CH2�TC��CH3��CHO���ʴ�Ϊ���ǻ���ȩ������ȥ��Ӧ��
��2��������������֪��X�Ľṹ��ʽ��CH3CH2CHO���ʴ�Ϊ��CH3CH2CHO��
��3��CH2�TC��CH3��COOH�ͼ״���Ӧ�IJ���ΪCH2�TC��CH3��COOCH3��������ͨ���Ӿ۷�Ӧ���ɸ߾����Ӧ����ʽΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��������������֪��E�Ľṹ��ʽ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��5��a��EΪ �����ǻ�������̼ԭ�����ڵ�̼ԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ����a����
�����ǻ�������̼ԭ�����ڵ�̼ԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ����a����
b�� ��CH2�TC��CH3��COOH������ȫ������Ӧ����M��1molM�к���4mol��������b��ȷ��
��CH2�TC��CH3��COOH������ȫ������Ӧ����M��1molM�к���4mol��������b��ȷ��
c��XΪCH3CH2CHO��YΪCH3CHO�����ߺ�����ͬ�Ĺ����š�����Ϊ������ṹ���ƣ����1��CH2ԭ���ţ���Ϊͬϵ���c��ȷ��
d��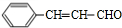 ��C=C˫����ͬһCԭ������2����ͬ�Ļ��ţ�����˳���칹�壬��d����
��C=C˫����ͬһCԭ������2����ͬ�Ļ��ţ�����˳���칹�壬��d����
�ʴ�Ϊ��bc��
��6��Y����D1����CH3CHO��3����HCHO������Ϣi��Ӧ���� ����Ӧ�Ļ�ѧ����ʽ��
����Ӧ�Ļ�ѧ����ʽ��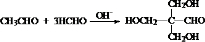 ���ʴ�Ϊ��
���ʴ�Ϊ��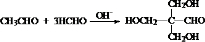 ��
��
��7��������������֪��Z�Ľṹ��ʽ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����Ϣiii��N�Ľṹ�������Ϣii�з�Ӧ��֪���γ�M������Ϊ
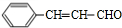 ��
�� ��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ
��C��E��Ӧ���Ժϳ�M�����M�ķ���ʽC21H28O8��֪��EΪ ����GΪ
����GΪ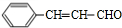 ��C��Eͨ��������Ӧ����M��
��C��Eͨ��������Ӧ����M����Y��E��ת�������E�Ľṹ��֪��Y�����������ٽ��G�Ľṹ��֪��������Z�к��б�������Ϸ�Ӧ��Ϣi��֪��YΪCH3CHO��ZΪ
 ����FΪ
����FΪ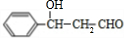 ��
����E�Ľṹ��֪��1����CH3CHO��3����HCHO������Ϣi��Ӧ����D��DΪ
 ��D�����������ӳɷ�Ӧ����E��
��D�����������ӳɷ�Ӧ����E�� ����
������1��������������֪��AΪHOCH2CH��CH3��CHO�������ǻ���ȩ����A��B��HOCH2CH��CH3��CHO������ȥ��Ӧ��ȥ1����H2O����CH2�TC��CH3��CHO���ʴ�Ϊ���ǻ���ȩ������ȥ��Ӧ��
��2��������������֪��X�Ľṹ��ʽ��CH3CH2CHO���ʴ�Ϊ��CH3CH2CHO��
��3��CH2�TC��CH3��COOH�ͼ״���Ӧ�IJ���ΪCH2�TC��CH3��COOCH3��������ͨ���Ӿ۷�Ӧ���ɸ߾����Ӧ����ʽΪ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����4��������������֪��E�Ľṹ��ʽ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����5��a��EΪ
 �����ǻ�������̼ԭ�����ڵ�̼ԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ����a����
�����ǻ�������̼ԭ�����ڵ�̼ԭ����û��Hԭ�ӣ����ܷ�����ȥ��Ӧ����a����b��
 ��CH2�TC��CH3��COOH������ȫ������Ӧ����M��1molM�к���4mol��������b��ȷ��
��CH2�TC��CH3��COOH������ȫ������Ӧ����M��1molM�к���4mol��������b��ȷ��c��XΪCH3CH2CHO��YΪCH3CHO�����ߺ�����ͬ�Ĺ����š�����Ϊ������ṹ���ƣ����1��CH2ԭ���ţ���Ϊͬϵ���c��ȷ��
d��
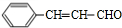 ��C=C˫����ͬһCԭ������2����ͬ�Ļ��ţ�����˳���칹�壬��d����
��C=C˫����ͬһCԭ������2����ͬ�Ļ��ţ�����˳���칹�壬��d�����ʴ�Ϊ��bc��
��6��Y����D1����CH3CHO��3����HCHO������Ϣi��Ӧ����
 ����Ӧ�Ļ�ѧ����ʽ��
����Ӧ�Ļ�ѧ����ʽ��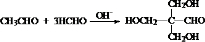 ���ʴ�Ϊ��
���ʴ�Ϊ��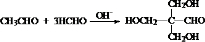 ��
����7��������������֪��Z�Ľṹ��ʽ��
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�����������⿼���л��ƶ���ϳɣ�ѧ��������ø����ķ�Ӧ��Ϣ���л���Ľṹ������ʽȷ��X��E��G�Ľ���ǹؼ����ѶȽϴ��Ƕ�ѧ����˼ά��������ѧ�����ȿ��飮

��ϰ��ϵ�д�
 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
�����Ŀ
