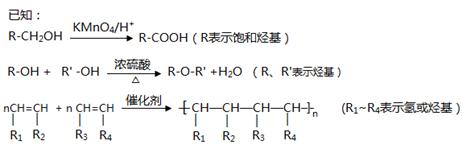
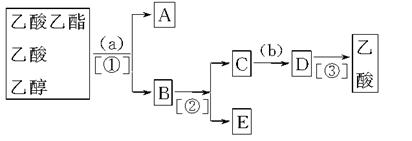
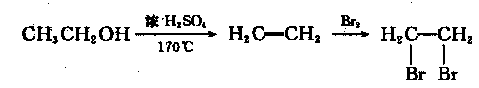��Ŀ����
�����£�һ������A��һ�ֵ�ϩ��B��ɵĻ�����壬A��B�������ֻ����4��̼ԭ�ӣ���B���ӵ�̼ԭ������A���ӵĶࡣ
��1����1L���������ȼ�գ���ͬ��ͬѹ�µõ�2.5LCO2���塣��ͨ�������ƶ�ԭ���������A��B���п��ܵ���ϼ�������ȣ�������������±���
| ��ϱ�� | A�ķ���ʽ | B�ķ���ʽ | A��B������ȣ�VA ��VB�� |
| �� | | | |
| �� | | | |
| �� | | | |
| �� | | | |
Ϊ��һ��ȷ��A��B����ʽ������ʵ�飺
��2��120��ʱ��ȡ1L�û��������9L������ϣ����ȼ�պ��ָ���120���ȼ��ǰ��ѹǿʱ�����������6.25������ͨ������ȷ��Ψһ���������A��B�ķ���ʽ��
��1��������ʽ��0.5�֣�����ȸ�1�֣���8�֣���ϱ�� A�ķ���ʽ B�ķ���ʽ A��B������ȣ�VA ��VB�� �� CH4 C3H6 1 ��3 �� CH4 C4H8 1 ��1 �� C2H6 C3H6 1 ��1 �� C2H6 C4H8 3 ��1
��2��C2H6��C4H8
���������������1����1�����������ȼ�պ�����2.5��CO2����B���ӵ�̼ԭ������A���ӵĶ࣬�������ֻ����̼ԭ����С��2.5��������CH4��C2H6����̼ԭ��������2.5��ϩ����C3H6��C4H8����ɣ����������ֿ��ܵ���ϣ�CH4��C3H6��CH4��C4H8��C2H6��C3H6��C2H6��C4H8������ÿһ�������������ϩ����̼ԭ������ȼ�պ����ɵ�CO2�������ȷ��A��B������ȣ��磺 ����V��CH4����V��C3H6����1��3���Դ����ƿɢ�CH4��C4H8��1��1��C2H6��C3H6��1��1��C2H6��C4H8��3��1��
����V��CH4����V��C3H6����1��3���Դ����ƿɢ�CH4��C4H8��1��1��C2H6��C3H6��1��1��C2H6��C4H8��3��1��
��2����1����̬���������ȼ�պ�����仯Ϊ��V������
CH4+2O2 CO2+2H2O������ ��V1=0������
CO2+2H2O������ ��V1=0������
C2H6+ O2
O2 2CO2+3H2O��������V2=0.5������
2CO2+3H2O��������V2=0.5������
C3H6+ O2
O2 3CO2+3H2O��������V3=0.5������
3CO2+3H2O��������V3=0.5������
C4H8+6O2 4CO2+4H2O��������V4=1.0������
4CO2+4H2O��������V4=1.0������
������ϵ�1������������������ȼ�գ��������Ϊ��Ϣ�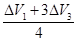 =0.375������
=0.375������
��Ϣ� =0.5������
=0.5������
��Ϣ� =0.5������
=0.5������
��Ϣ� =0.625������
=0.625������
�� ��100%=6.25%
��100%=6.25%
����Ϣܷ������⣬��A��C2H6��B��C4H8��
���㣺�����л��ﻯѧʽȷ�����йؼ���

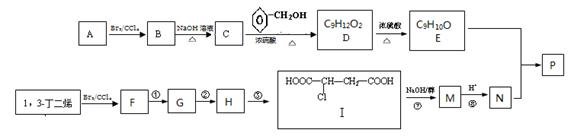
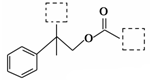
�Լ�����������( )�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����
)�����ںϳ�ҩ����м��塣���������ת����ϵ�ش��й�����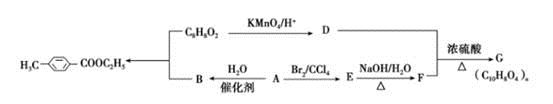
(1)D�к��й����ŵ�������________��A��E�ķ�Ӧ����Ϊ______��
(2)G�Ľṹ��ʽΪ_______��
(3)д��1�����������ұ�����ֻ��һ��ȡ������C8H8O2��ͬ���칹�� ��
(4)�����( )�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
)�ǶԼ�������������ͬ���칹�壬�������������ܷ�����Ӧ����________(�����)��
| A��NaOH��Һ | B��NaHCO3��Һ | C��KMnO4/H+ | D��FeCl3��Һ |
��������̬���Ļ�����֪�����Ƕ���ʹ��ˮ��ɫ���ҷ�����̼ԭ������С��5��1����û��������ȫȼ�պɵõ�3.6���������̼��3���ˮ����(�����������ͬ��ͬѹ�²ⶨ)
(1)�����������������������________��
| A���飬ϩ | B��ϩ��ϩ | C��ϩ��Ȳ | D��Ȳ��Ȳ |
(2)ͨ������ȷ�����������ķ���ʽ�Լ������ڻ�����е�����ȡ�
��ˮ�����Ǻϳ����������Ȼ�������Ҫ���裬ij�����ϳ�·�����£�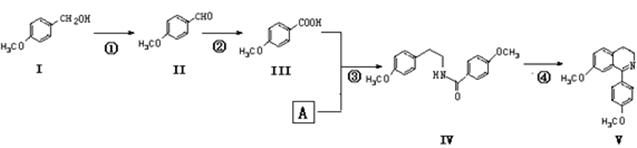
��1���������Ļ�ѧʽΪ ��
��2��������A�Ľṹ��ʽΪ ����3����Ӧ�ٵĻ�ѧ����ʽΪ ��
��4������˵��������� ��
| A����������ܷ���������Ӧ | B��������������ڷ����� |
| C����Ӧ������������Ӧ | D�������������4 molH2�����ӳɷ�Ӧ |
��5����������뻯�����Ϊͬ���칹�壬���к���������������FeCl3��Һ������ɫ��Ӧ���䱽���ϵ�һ�ȴ���ֻ��2�֡�д��һ���������������Ģ��Ľṹ��ʽ�� ��
��6�����������ͼ��ʾ����һ��������Ҳ�ܷ������������ڢܲ��Ļ�����Ӧ���������������Ӧ����Ľṹ��ʽΪ ��
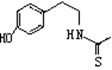
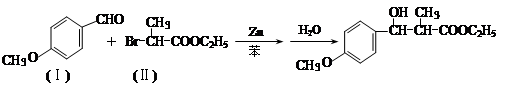
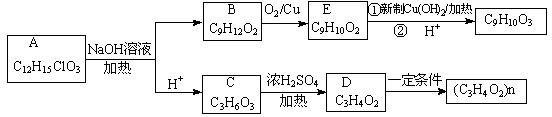
 ͨ������·�߿ɺϳ�(��)��
ͨ������·�߿ɺϳ�(��)��
 �����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�ķ�Ӧ������ ��
�����ɣ���ʱ�����ܵõ���һ�ָ�����C6H8O4���÷�Ӧ�Ļ�ѧ����ʽΪ ���÷�Ӧ�ķ�Ӧ������ �� Ҳ�����л���(��) (����)�������Ʒ�Ӧ�ٵ�ϵ�з�Ӧ���������л���Ľṹ��ʽΪ ��
Ҳ�����л���(��) (����)�������Ʒ�Ӧ�ٵ�ϵ�з�Ӧ���������л���Ľṹ��ʽΪ ��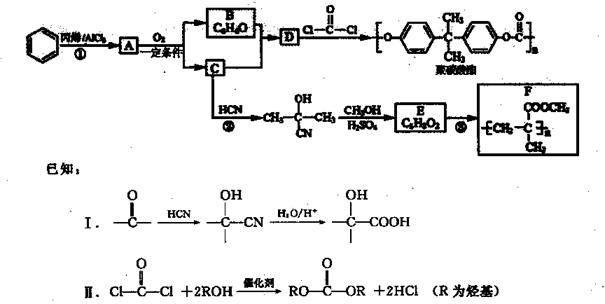
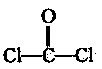 ��Ӧ�ϳɾ�̼���Ļ�ѧ����ʽ ��
��Ӧ�ϳɾ�̼���Ļ�ѧ����ʽ ��