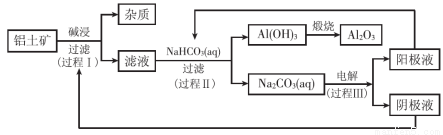
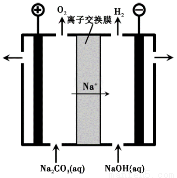
题目内容
已知
①H2(g)+1/2O2(g)=H2O(l) △H =-284.2kJ /mo1
②2NH3(g)+3/2O2(g)=N2(g)+3 H2O(l) △H =-760.2 kJ /mo1
则可推知1/2 N2(g)+ 3/2H2(g)= NH3(g) 的△H是( )
A.-91.96 kJ/mol B.-104.5 kJ/mol
C.+476.52 kJ/mol D.-46.2 kJ/mol
练习册系列答案
 口算题卡加应用题集训系列答案
口算题卡加应用题集训系列答案 综合自测系列答案
综合自测系列答案
相关题目



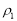
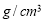 质量分
质量分 数是
数是 的浓盐酸,与水配制成体积比为1:4的稀盐酸,密度为
的浓盐酸,与水配制成体积比为1:4的稀盐酸,密度为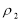
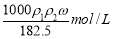 B.
B.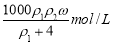
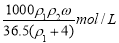 D.
D.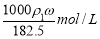
 SO2(g)放出的热量大于297.23 kJ
SO2(g)放出的热量大于297.23 kJ SiHCl3+H2;
SiHCl3+H2;