��Ŀ����
ij��ѧС���ͬѧ��ʵ����ѧϰ����ʵ�����ϰ�������������
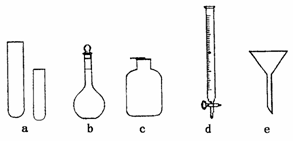 |
��1��ָ����ʦҪ��ͬѧ��д�����������ƣ���ͬѧ��д�Ĵ����±��������ҳ����еĴ����������������д���±��У�����ȷ����ÿղ���Ҫ��д����
������� | a | b | c | d | e |
���� | �Թ� | ����ƿ | ����ƿ | ��ʽ�ζ��� | ��ͨ©�� |
���������� |
��2������e����;����ͬѧ˵����ɷ�����װ�á��㻹��˵������������;��
���������������������������������� ���������������������������������� ��
������ ���������������������������������������������������������������� ��
 ��3����ͬѧ������ͼװ���Դ���ʯ��ϡ���ᷴӦ��ȡCO2����ʦָ��������Ҫ̫���ϡ���ᣬ����˷ѡ���ͬѧѡ���������һ������������װ���ϣ������������⡣����Ѹ���������ͼ�к��ʵ�λ�á�
��3����ͬѧ������ͼװ���Դ���ʯ��ϡ���ᷴӦ��ȡCO2����ʦָ��������Ҫ̫���ϡ���ᣬ����˷ѡ���ͬѧѡ���������һ������������װ���ϣ������������⡣����Ѹ���������ͼ�к��ʵ�λ�á�
������� |
| b |
| d |
|
���� |
|
|
|
|
|
���������� |
| ����ƿ |
| ��ʽ�ζ��� |
|
��ÿ��1�֣���2�֡���д��д��һ����1�֣�����Ϊֹ��
��2������ϸ�������м�Һ�壻����ɹ���װ�ã�ֻҪ����������ɣ�������������ɣ���ÿ�����1�֣�
 |
��3����2�֣�
��ֻ�軭���Թܵ�λ�ü��ɵ÷֣�

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�����6�֣������������ʣ� ��NaCl���塡��Һ̬SO2���۴����ᡡ�����ᱵ����ͭ �ƾ���C2H5OH�� ���ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ����������������������������������������������������
��2������������ʵ���������������������������������������
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ��������������������������������
����4�֣�
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð�
������ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��
A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ��
��ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ
������¼���£�
| ʵ����� | ��һ�� | �ڶ��� | ������ |
| ����NaOH��Һ���/mL | 26.02 | 25.35 | 25.30 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ�
��ԭ�������������������������
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
C����һ�εζ��õ���ƿδ��ϴ
D���ζ�����ʱ�����Ӷ���
��3�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ(���ػ���)��
c������������ ������ ��
����15�֣�
��֪����25ʱH2OH++OH-��KW=10-14�� CH3COOH
H++ CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH��
�ı�ֵ�� ���������С�����䡱��
��2��������ˮ������ӷ���ʽΪ�������������������������¶�ʱ��C(OH��)����������
���������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д�����
�ı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵ
Ϊ������������������ ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������pH��3��HA��ҺV1mL��pH��11��NaOH{��ҺV2 mL����϶��ã�������˵������ȷ����____________��
A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1
B����V1=V2����Ӧ����ҺpHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2
��7����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ0.01mol��L-1�������м����
������ƺ�����Һ��C(OH-)Ϊ2.2��10-5mol��L-1���������ֽ���������
�������� �����ɳ�����ԭ�������� ��
��KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��KSP��Cd��OH��2��=2.5��10-14��
��8��ȡ10mL0.5mol��L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��
=������ ����
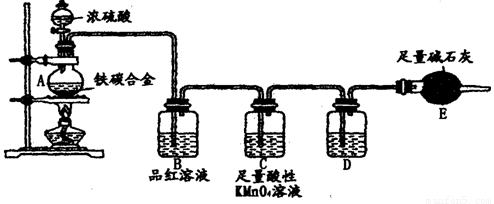
[2012�������ʼ�]��11�֣�ij�о�ѧϰС��Ϊ̽��Fe3+�����Ƿ�������SO2����������µ�ʵ��װ�ã�����ʵ��������װ�õ����������ã���
��1����ͬѧ��������ʵ�߿���װ����ȡSO2������̽��ʵ�顣
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��װ��B�������� ��
�۷�Ӧһ��ʱ���ͬѧȡװ��C��������Һ�������м���HCl�ữ��BaCl2��Һ�۲쵽�а�ɫ�����������ɴ����ó����ۣ�Fe3+������SO2��
��2����ͬѧ��Ϊ��ͬѧ��ʵ�鲻�Ͻ��������������߿���װ�����װ��A����ʹװ��E���Լ���Ӧһ��ʱ��رջ���1������2���ַ�ӦƬ�̺�ȡװ��C��������Һ�������м�������KMnO4��Һ���۲쵽KMnO4��Һ�Ϻ�ɫ��ȥ���ɴ����ó����ۣ�Fe3+�ѱ�SO2��ԭ��Fe2+��
�ٸ�ʵ����H2ʱ����Ũ��������450mL3mol/L��ϡ���ᣬ����������IJ����������ձ�����Ͳ������������ͷ�ι��⣬���� ��
���ƹ�������������������û��ϴ���ձ��벣����������������Һ��Ũ�Ȼ� ���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
���ڸ�ʵ�������H2����������� ��
��3����ͬѧ��Ϊ�ס��ҵ�ʵ������Ͻ���Ϊ����������ͬѧ��ʵ��װ�ü�����������ʵ�顣��װ��F�ڷ�Ӧһ��ʱ���ȡװ��C��������Һ�������м���HCl�ữ��BaCl2��Һ���ó����ͬѧ��ͬ�Ľ��ۡ���������ش�
�ټ�ͬѧʵ�鲻�Ͻ���ԭ���� ����ѡ����ţ�
| A��SO2�ܽ���̫С |
| B��SO2����Fe3+������Ӧ |
| C��H2SO3��BaCl2����Ӧ |
| D��װ���еĿ�����SO2����ˮ��Ҳ������H2SO4 |
����6�֣������������ʣ� ��NaCl���塡��Һ̬SO2���۴����ᡡ�����ᱵ����ͭ �ƾ���C2H5OH�� ���ۻ���KCl����NaOH��Һ��
�����������ʻش��������⡣������ţ�
��1��������״̬���ܵ�������������������������������������������������� ��
��2������������ʵ������������������������������������� ��
��3�����ڷǵ���ʣ�������ˮ���ˮ��Һ�ܵ������������������������������ ��
����4�֣�
ij��ѧʵ��С��̽������ʳ�ð״��д���ĵ�ȷŨ�ȣ�ȡ25.00mLijƷ��ʳ�ð�
������ƿ�У���ʵ������Ũ��Ϊcb mol/L�ı�NaOH��Һ������еζ���
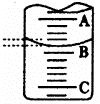
��1����ͼ��ʾ50mL�ζ�����Һ���λ�ã���A��C�̶ȼ����l mL��
A���Ŀ̶�Ϊ25���ζ�����Һ�����ӦΪ����������mL��
��2��Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ��
��ȡ�״������ΪVmL��NaOH��ҺŨ��Ϊc mo1/L������ʵ
������¼���£�
|
ʵ����� |
��һ�� |
�ڶ��� |
������ |
|
����NaOH��Һ���/mL |
26.02 |
25.35 |
25.30 |
���ϱ����Կ�������һ��ʵ���м�¼����NaOH��Һ��������Զ��ں����Σ�
��ԭ����������������������� ��
A���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B��ʢװ��Һ�ĵζ���װҺǰ������ˮ��ϴ����δ�ñ�Һ��ϴ
C����һ�εζ��õ���ƿδ��ϴ
D���ζ�����ʱ�����Ӷ���
��3�������������ݣ�д������ð״��д�������ʵ���Ũ�ȵı���ʽ(���ػ���)��
c������������ ������ ��
����15�֣�
��֪����25ʱH2O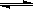 H++OH-��KW=10-14�� CH3COOH
H++OH-��KW=10-14�� CH3COOH
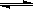 H++ CH3COO����Ka=1.8��10-5
H++ CH3COO����Ka=1.8��10-5
��1��ȡ����������Һ���������������ƹ��壬��ʱ��Һ��C��H+����C��CH3COOH��
�ı�ֵ�� �� �������С�����䡱��
��2��������ˮ������ӷ���ʽΪ���������������� ���������¶�ʱ��C(OH��)����������
���������С�������䡱����
��3��0��5mol��L-1��������ҺpHΪm����ˮ��ij̶ȣ���ˮ��Ĵ�������ԭ�д�����
�ı�ֵ��Ϊa��1mol��L-1��������ҺpHΪn��ˮ��ij̶�Ϊb����m��n�Ĺ�ϵ
Ϊ������������������ ��a��b�Ĺ�ϵΪ����������������ڡ���С�ڡ������ڡ�����
��4�����������Ũ�ȵĴ��������������Һ��Ϻ�������Һ������Ũ���ɴ�С��˳�������������������� ������ ��
��5�������������������Һ��Ϻ�pH<7����c��Na+��_______________ c��CH3COO����������ڡ�����С�ڡ����ڡ�����
��6������pH��3��HA��ҺV1mL��pH��11��NaOH{��ҺV2 mL����϶��ã�������˵������ȷ����____________��
A������Ӧ����Һ�����ԣ���c��H+��+c��OH������2��10��7mol��L��1
B����V1=V2����Ӧ����ҺpHһ������7
C������Ӧ����Һ�����ԣ���V1һ������V2
D������Ӧ����Һ�ʼ��ԣ���V1һ��С��V2
��7����ij��Һ�к�Mg2+��Cd2+��Zn2+�������ӵ�Ũ�Ⱦ�Ϊ0.01mol��L-1�������м����
������ƺ�����Һ��C(OH-)Ϊ2.2��10-5mol��L-1���������ֽ���������
�������� �����ɳ�����ԭ�������� ��
��KSP��Mg��OH��2��=1.8��10-11��KSP��Zn��OH��2��=1.2��10-17��KSP��Cd��OH��2��=2.5��10-14��
��8��ȡ10mL0.5mol��L-1������Һ����ˮϡ�͵�500mL�������Һ����ˮ�������c��H+��
=������ ����