��Ŀ����
( 12��)��1����һ�ܱյ�2L�����������8mol SO2��4mol 18O2����һ�������¿�ʼ��Ӧ��2SO2��g����O2��g�� 2SO3��g��2minĩ�����������7.2mol SO2���Իش�
2SO3��g��2minĩ�����������7.2mol SO2���Իش�
�� ��Ӧ��18Oԭ�Ӵ�������Щ������������������������������������������
�� 2minĩSO3��Ũ��________________________��
�� ��O2��Ũ�ȱ仯��ʾ��ʱ����ڵĻ�ѧ��Ӧ����_______________________��
��ij��ѧ��Ӧ2A (g)  B(g)+D(g)��3�ֲ�ͬ�����½��У�B��D����ʼŨ��Ϊ0����Ӧ��A��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±���
B(g)+D(g)��3�ֲ�ͬ�����½��У�B��D����ʼŨ��Ϊ0����Ӧ��A��Ũ�ȣ�mol/L���淴Ӧʱ�䣨min���ı仯������±���
|
ʵ����� |
|
0 |
10 |
20 |
30 |
40 |
50 |
60 |
|
1 |
800�� |
1.0 |
0.80 |
0.67 |
0.57 |
0.50 |
0.50 |
0.50 |
|
2 |
800�� |
C2 |
0.92 |
0.75 |
0.63 |
0.60 |
0.60 |
0.60 |
|
3 |
820�� |
1.0 |
0.40 |
0.25 |
0.20 |
0.20 |
0.20 |
0.20 |
�����������ݣ����������գ�
(1) ʵ��1�ﵽƽ���ʱ����__________min��C2_____1.0 min��L-1(���������������)��
(2)ʵ��3��ʵ��1�ķ�Ӧ����_________(��족������)��ԭ����___________________________________________________________________________��
 (3) ���2A
(g) B(g)+D(g)��һ�����ȷ�Ӧ����ôʵ��3��ʵ��1��ȣ�����ͬ���ʱ___________���յ������࣬������___________________________________________��
(3) ���2A
(g) B(g)+D(g)��һ�����ȷ�Ӧ����ôʵ��3��ʵ��1��ȣ�����ͬ���ʱ___________���յ������࣬������___________________________________________��
��
��1��40��1�֣� ������1�֣� ��2���죨1�֣��� ʵ��3��ʵ��1���¶ȸߣ���Ӧ���ʿ죨2�֣� ��3��ʵ��3��A��ת���ʴ�Щ���ֽ��A��Щ��������Ӧ���������ࡣ��2�֣�
����������1���� ��Ϊ�ǿ��淴Ӧ���������������ͬʱ����������Ҳ�Ƿֽ�ģ����Է�Ӧ��18Oԭ�Ӵ�����O2��SO2��SO3�С�
��2minĩ�����������7.2mol SO2��������SO2��8mol��7.2mol��0.8mol��������������������0.8mol����Ũ����0.8mol��2L��0.4mol/L��
������������0.4mol�����������ķ�Ӧ������ ��
��
��1��ʵ��1�е���Ӧ���е�40min�����ʵ�Ũ�Ȳ��ٷ����仯�����Է�Ӧ�ﵽƽ��״̬���������ŷ�Ӧ�Ľ��У���Ӧ�������ͣ�������C2��0.92��0.92��0.75������C2��1.0mol/L��
��2������ʵ��3��ʵ��1���¶ȸߣ���˷�Ӧ���ʿ졣
��3����������Ӧʱ���ȷ�Ӧ������ʵ��3��A��ת���ʴ�Щ���ֽ��A��Щ�����������Ӧ���������ࡣ

 �϶�����̽��������2013��12��2���賿1��30�����Ĵ�ʡ�������Ƿ�������ʹ�ó�����������ǿ�����ػ������ɹ��������һ�Ӽ��Ͷ��Ӽ�ʹ��ƫ�����º�N2O4��Ϊ�ƽ�������ӦʽΪ��CH3��2NNH2+2N2O4�T2CO2+4H2O+3N2�����Ӽ���ʹ��Ч�ܸ��ߵ�Һ�⣨H2����Һ����O2��������˵����ȷ���ǣ�������
�϶�����̽��������2013��12��2���賿1��30�����Ĵ�ʡ�������Ƿ�������ʹ�ó�����������ǿ�����ػ������ɹ��������һ�Ӽ��Ͷ��Ӽ�ʹ��ƫ�����º�N2O4��Ϊ�ƽ�������ӦʽΪ��CH3��2NNH2+2N2O4�T2CO2+4H2O+3N2�����Ӽ���ʹ��Ч�ܸ��ߵ�Һ�⣨H2����Һ����O2��������˵����ȷ���ǣ�������| A��N2O4�ڷ�Ӧ�б����� | B����CH3��2NNH2���л�ԭ�� | C����Ӧ��1mol N2O4�õ�4mol e- | D��Һ����Һ���ķ�Ӧ�У�Һ����Һ���������Ϊ 2��1 |
����12�֣�����250mL 1.0mol/L NaOH��Һ����ش��������⣺
�������������У�A ������ƽ B ��Ͳ C�ձ� D ������ E ©�� F 500mL����ƿ
G ҩ�� H 250mL����ƿ I ��ͷ�ι� J ����
��Ҫ�õ���������
������NaOH��������Ϊ ��
�����Ƶ�ʵ�鲽�����£�
�ټ��� �ڳ��� ���ܽ� ��ת�ơ�ϴ�� �ݶ��� ��ҡ��
���еڢۡ��ܡ��ݲ�ʵ�������Ҫ�õ������������÷ֱ��� �� ��
��
��������ƿ��ȷ����Һ����Ĺ����У���ɺ��ڼ�������ˮ��������
��
���и����У����ܵ���ʵ��Ũ��ƫ�ߵ��� �����ţ�
| A������ƽ�����ϵ�ֽ����NaOH����ֽ�ϳ��� |
| B��NaOH�ܽ�ʱ�ų��������ȣ�δ��ȴ����������Һ |
| C���ܽ�NaOH����֮����ձ�δϴ�� |
| D��������ƿ��ת��Һ��ʱ�������� |
�ʽ�ȡ����10mL��Һ��ˮϡ�͵�100mL��ϡ�ͺ���Һ��N
 aOH�����ʵ���Ũ��Ϊ ��
aOH�����ʵ���Ũ��Ϊ �� 
 ��235U ��
��235U ��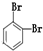
 ��12��
��12��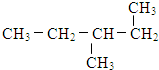
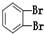
 ���ַ����У�̼ԭ��Ϊsp2�ӻ��ķ�����_______________________________________________��
���ַ����У�̼ԭ��Ϊsp2�ӻ��ķ�����_______________________________________________�� ����_______mol���γ�
����_______mol���γ� ����_______mol��
����_______mol��