��Ŀ����
�����������������ƽ�ȼ�ϵ�ص��з���ȼ�ϵ�ص�ȼ�����������״��ȣ���1��ͨ��������--��ѭ�����տ��Ƶ�ȼ�ϵ�ص�ȼ��������
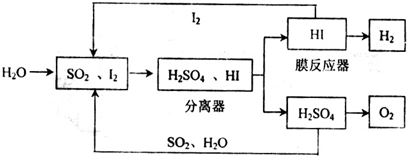
�ٸ�ѭ�����չ��̵��ܷ�Ӧ����ʽΪ
���û�ѧƽ���ƶ���ԭ����������HI�ֽⷴӦ��ʹ��Ĥ��Ӧ�������H2��Ŀ����
��2��������̼�ǵ�������ЧӦ��������ף�Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ����������Ӧ�ϳɼ״�����֪�������״�ȼ�յ��Ȼ�ѧ����ʽ���£�
2H2��g��+O2��g��=2H2O��l������H=-283.0kJ?mol-1����
2CH3OH��1��+3O2��g����2CO2��g��+4H2O��1������H=-726.0kJ?mol-1����
д��������̼�������ϳɼ״�Һ����Ȼ�ѧ����ʽ
��3���״�--����ȼ�ϵ��������ϡ��������������Ϊ�������ʣ�����ϡ�������������ڸ������ܴ���O2-��
�ٸ��������ķ�Ӧ��
����ϡ������������Ĺ��������У�O2-�����ƶ�������
��4����֪��Ӧ2CH30H?CH3OCH3��g��+H2O��g��ij�¶��µ�ƽ�ⳣ��Ϊ400���� �¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol?L-1�� | 0.44 | 0.6 | 0.6 |
��������CH3OH��10min��Ӧ�ﵽƽ�⣬��ʱ���ڷ�Ӧ����v��CH30H��=
��������1����д���������еĸ���Ӧ����ʽ��Ȼ��д���ܵķ�Ӧ����ʽ���ɣ�
�ڸ���ƽ���ƶ���ԭ�����з�����
��2�����ݸ�˹���ɺ��Ȼ�ѧ����ʽ����õ�������̼�������ϳɼ״�Һ����Ȼ�ѧ����ʽ��
��3����ȼ�ϵ��������ԭ��ع���ԭ���������������õ����ӷ�����ԭ��Ӧ��������ǹ��壬�����õ��������������ӣ��״��ڸ���ʧ���ӷ���������Ӧ���ɶ�����̼��
�ڸ���ԭ��������������������жϣ�
��3���ٽ������ʵ�Ũ�ȴ���ƽ�ⳣ������ʽ������������ƽ�ⳣ�����бȽϣ��Ӷ��ж�ƽ���ƶ�����
������ﵽƽ��ʱ���ѵ����ʵ���Ũ�ȣ�Ȼ������ƽ�ⳣ��������ﵽƽ��ʱ���ѵ�Ũ�ȣ��ټ����10min�ڵķ�Ӧ����v��CH30H����
�ڸ���ƽ���ƶ���ԭ�����з�����
��2�����ݸ�˹���ɺ��Ȼ�ѧ����ʽ����õ�������̼�������ϳɼ״�Һ����Ȼ�ѧ����ʽ��
��3����ȼ�ϵ��������ԭ��ع���ԭ���������������õ����ӷ�����ԭ��Ӧ��������ǹ��壬�����õ��������������ӣ��״��ڸ���ʧ���ӷ���������Ӧ���ɶ�����̼��
�ڸ���ԭ��������������������жϣ�
��3���ٽ������ʵ�Ũ�ȴ���ƽ�ⳣ������ʽ������������ƽ�ⳣ�����бȽϣ��Ӷ��ж�ƽ���ƶ�����
������ﵽƽ��ʱ���ѵ����ʵ���Ũ�ȣ�Ȼ������ƽ�ⳣ��������ﵽƽ��ʱ���ѵ�Ũ�ȣ��ټ����10min�ڵķ�Ӧ����v��CH30H����
����⣺���ڷ�Ӧ���з�����Ӧ��SO2+I2+2H2O=2HI+H2SO4��2H2SO4=2SO2+2H2O+O2����Ĥ��Ӧ���еķ�ӦΪ��2HI?I2+H2���������Ϸ�Ӧ����ʽ�ɵã�2H2O=O2+2H2��
�ʴ�Ϊ��2H2O=O2+2H2��
����Ĥ�������з�����Ӧ��2HI?I2+H2����H2�������������ƽ�������ƶ�������I2��H2�����ɣ�
�ʴ�Ϊ��������ƽ�������ƶ��������ڵ�����������ɣ�
��2��2H2��g��+O2��g��=2H2O��l������H=-283.0kJ?mol-1����
2CH3OH��l��+3O2��g����2CO2��g��+4H2O��l����H=-726.0kJ?mol-1 ��
���ݸ�˹���ɢ١�3-�ڵõ���2CO2 ��g��+6H2 ��g��=2CH3OH��l��+2H2O ��l����H=-123kJ?mol-1��
�Ȼ�ѧ����ʽΪ��CO2 ��g��+H2 ��g��=CH3OH��l��+H2O ��l����H=-61.5kJ?mol-1��
�ʴ�Ϊ��CO2 ��g��+H2 ��g��=CH3OH��l��+H2O ��l����H=-61.5kJ?mol-1��
��3���ټ״�һ����ȼ�ϵ���������õ����ӷ�����ԭ��Ӧ���������ӣ��缫��ӦΪ��O2+4e-=2O2-�� �״��ڸ���ʧ���ӷ���������Ӧ���缫��ӦΪ��CH3OH+3O2--6e-=CO2+2H2O��
�ʴ�Ϊ��CH3OH+3O2--6e-=CO2+2H2O��
�����ݵ缫��Ӧ������֪ԭ����������������������Ӵ����������ƶ���
�ʴ�Ϊ��������������
���ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K=
��������Ũ�ȴ���ƽ�ⳣ������ʽ��
=1.86��400���ʷ�Ӧ������Ӧ������У�����Ӧ���ʴ����淴Ӧ���ʣ�
�ʴ�Ϊ������
����ﵽƽ��ʱ���ѵ����ʵ���Ũ��Ϊx��
2CH3OH��g��?CH3OCH3��g��+H2O��g��
ijʱ��Ũ�ȣ�mol?L-1����0.44 0.6 0.6
ת��Ũ�ȣ�mol?L-1����2x x x
ƽ��Ũ�ȣ�mol?L-1����0.44-2x 0.6+x 0.6+x
�������Ũ�ȴ���ƽ�ⳣ������ʽ�ɵã�K=
=400����� x=0.2mol/L��
�ﵽƽ��ʱ���ѵ�Ũ��Ϊ��0.8mol/L����10minת���ļ״������ʵ���Ũ��Ϊ��c��CH3OH��=2c��CH3OCH3��=1.6mol/L��
���Լ״��ķ�Ӧ����Ϊv��CH3OH��=
=0.16 mol/��L?min����
�ʴ�Ϊ��0.16 mol/��L?min����
�ʴ�Ϊ��2H2O=O2+2H2��
����Ĥ�������з�����Ӧ��2HI?I2+H2����H2�������������ƽ�������ƶ�������I2��H2�����ɣ�
�ʴ�Ϊ��������ƽ�������ƶ��������ڵ�����������ɣ�
��2��2H2��g��+O2��g��=2H2O��l������H=-283.0kJ?mol-1����
2CH3OH��l��+3O2��g����2CO2��g��+4H2O��l����H=-726.0kJ?mol-1 ��
���ݸ�˹���ɢ١�3-�ڵõ���2CO2 ��g��+6H2 ��g��=2CH3OH��l��+2H2O ��l����H=-123kJ?mol-1��
�Ȼ�ѧ����ʽΪ��CO2 ��g��+H2 ��g��=CH3OH��l��+H2O ��l����H=-61.5kJ?mol-1��
�ʴ�Ϊ��CO2 ��g��+H2 ��g��=CH3OH��l��+H2O ��l����H=-61.5kJ?mol-1��
��3���ټ״�һ����ȼ�ϵ���������õ����ӷ�����ԭ��Ӧ���������ӣ��缫��ӦΪ��O2+4e-=2O2-�� �״��ڸ���ʧ���ӷ���������Ӧ���缫��ӦΪ��CH3OH+3O2--6e-=CO2+2H2O��
�ʴ�Ϊ��CH3OH+3O2--6e-=CO2+2H2O��
�����ݵ缫��Ӧ������֪ԭ����������������������Ӵ����������ƶ���
�ʴ�Ϊ��������������
���ٸ÷�Ӧ��ƽ�ⳣ������ʽΪ��K=
| c(CH3OCH3)?c(H2O) |
| c2(CH3OH) |
| 0.6��0.6 |
| 0.442 |
�ʴ�Ϊ������
����ﵽƽ��ʱ���ѵ����ʵ���Ũ��Ϊx��
2CH3OH��g��?CH3OCH3��g��+H2O��g��
ijʱ��Ũ�ȣ�mol?L-1����0.44 0.6 0.6
ת��Ũ�ȣ�mol?L-1����2x x x
ƽ��Ũ�ȣ�mol?L-1����0.44-2x 0.6+x 0.6+x
�������Ũ�ȴ���ƽ�ⳣ������ʽ�ɵã�K=
| (0.6+x)2 |
| (0.44-2x)2 |
�ﵽƽ��ʱ���ѵ�Ũ��Ϊ��0.8mol/L����10minת���ļ״������ʵ���Ũ��Ϊ��c��CH3OH��=2c��CH3OCH3��=1.6mol/L��
���Լ״��ķ�Ӧ����Ϊv��CH3OH��=
| 1.6mol/L |
| 10min |
�ʴ�Ϊ��0.16 mol/��L?min����
���������⿼���Ϊ�ۺϣ������ϴ���Ŀ�Ѷ��еȣ�ע�⡰ʼ��ת��ƽ���ǽ���йػ�ѧƽ��ġ������ۡ����ⷨ����������һ��ȷ�������Խ���й�ƽ��ʱ��ƽ�ⳣ�����㡢ת���ʡ���Ӧ���ʡ�ƽ��ʱ�ɷֵ���������ȣ�

��ϰ��ϵ�д�
�����Ŀ
 Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ����������Ӧ�ϳɼ״����״�������ȼ�ϵ�ص���Ҫȼ�ϡ���֪�������״�ȼ�յ��Ȼ�ѧ����ʽ���£�
Ŀǰ���Ǵ���������̼�ķ���֮һ��ʹ����������Ӧ�ϳɼ״����״�������ȼ�ϵ�ص���Ҫȼ�ϡ���֪�������״�ȼ�յ��Ȼ�ѧ����ʽ���£�