��Ŀ����
(14��)2014��10�³����������������Ű�ӱ���������ȵ��������У�ȼú������β������ɿ�����Ⱦ��ԭ��֮һ��
��1������β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g)  2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
�ٸ÷�Ӧ��ƽ�ⳣ������ʽ ��
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� ������ţ���


��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣úȼ�ղ����������������������CH4����ԭNOx�������������������Ⱦ��
��֪��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g) ��H����867 kJ/mol
2NO2(g) N2O4(g)
��H����56.9 kJ/mol
N2O4(g)
��H����56.9 kJ/mol
H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ�� ��
��3����һ�������£�Ҳ������NH3����NOx����֪NO��NH3������Ӧ����N2��H2O������NO��NH3�Ļ����1mol����ַ�Ӧ��õ��Ļ�ԭ��������������1.4 g����ԭ��Ӧ�������NO�����ʵ���������_____________��
��4���Լ���Ϊԭ����ȡ�����ǹ�ҵ�ϳ��õ����ⷽ������2 molCH4������H2O��g����Ӧ��������_______mol H2��д���÷�Ӧ�Ļ�ѧ����ʽ_________________________________________________��
(5)���������Ƶõ�H2���Ժ�CO��һ�������ºϳɼ״��Ͷ����ѣ�CH3OCH3���������������ʡ������������ʵ���1:1����Ӧ����ԭ�������ʴ�100%���ϳɵ����ʿ����� ��
a.���� b.�״� c.��ȩ d.����
��1����K��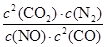 ��2�֣� ��bd ��2�֣�
��2�֣� ��bd ��2�֣�
��2��CH4(g)+N2O4(g)��N2(g) +2H2O(l) + CO2(g) ��H����898.1kJ/mol ��2�֣�
��3��0.3mol��0.8mol��2�֣�
��4��8 mol ��2�֣� CH4��g��+2 H2O��g���� CO2��g��+4H2��g����2�֣� ��5��c��2�֣�
��������
�����������1���ٻ�ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ����˸��ݷ�Ӧʽ2NO(g) + 2CO(g)  2CO2(g)+ N2(g)��֪���÷�Ӧ��ƽ�ⳣ������ʽK��
2CO2(g)+ N2(g)��֪���÷�Ӧ��ƽ�ⳣ������ʽK��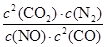 ��
��
��a������ƽ���������������ȣ����ٱ仯��t1ʱ��V�����֮���淴Ӧ�������ʷ����仯��δ����ƽ�⣬��a����b���÷�Ӧ����ӦΪ���ȷ�Ӧ���淴Ӧ�����¶����ߣ���ѧƽ�ⳣ����С������ƽ����¶�Ϊ��ֵ������ߣ�ƽ�ⳣ�����䣬Ϊ��С��ͼ����ʵ�ʷ��ϣ���b��ȷ��c��t1ʱ�̺������̼��NO�����ʵ��������仯��t1ʱ��δ����ƽ��״̬����c����d�����ŷ�Ӧ�Ľ��У�NO������������С��t1ʱ��NO����������Ϊ��ֵ�����ٷ����仯��˵����Ӧ����ƽ��״̬����d��ȷ����ѡbd��
��2�����ݷ�Ӧ��CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)�� ��H����867 kJ/mol��
��2NO2(g) N2O4(g)
��H����56.9 kJ/mol����H2O(g) �� H2O(l)
��H �� ��44.0 kJ��mol�����ݸ�˹���ɿ�֪���٣��ڣ��ۡ�2���õ���ӦCH4(g)+N2O4(g)��N2(g) +2H2O(g) + CO2(l)
�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H����867 kJ/mol��56.9
kJ/mol��44.0 kJ��mol��2����898.1kJ/mol��
N2O4(g)
��H����56.9 kJ/mol����H2O(g) �� H2O(l)
��H �� ��44.0 kJ��mol�����ݸ�˹���ɿ�֪���٣��ڣ��ۡ�2���õ���ӦCH4(g)+N2O4(g)��N2(g) +2H2O(g) + CO2(l)
�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H����867 kJ/mol��56.9
kJ/mol��44.0 kJ��mol��2����898.1kJ/mol��
��3��������NO��Ӧ�Ļ�ѧ����ʽΪ6NO��4NH3=5N2��6H2O�����а����ǻ�ԭ����NO�������������������������Ҳ�ǻ�ԭ�����ԭ��������������1mol��������28g����
6NO��4NH3=5N2��6H2O ��ԭ��������������
6mol 4mol 28g
0.3mol 0.2mol 1.4g
�������NO��������ԭ��Ӧ�������NO�����ʵ�����1.0mol��0.2mol��0.8mol
���������������ԭ��Ӧ�������NO�����ʵ�����0.3mol
��4��CH4������H2O��g����ӦҪ����������������Ϊ������Ӧ����������CO2����Ӧ�Ļ�ѧ����ʽΪCH4��g��+2 H2O��g���� CO2��g��+4H2��g����.����2mol����������ˮ������Ӧ�������8mol������
��5����H2��CO�����ʵ���1:1����Ӧ����ԭ�������ʴ�100%ʱ������ԭ���غ��֪������Ļ�ѧʽӦ����CH2O���������Ǽ�ȩ����ѡc��
���㣺����ƽ�ⳣ����ƽ��״̬���жϣ���˹���ɵ�Ӧ�ã�������ԭ��Ӧ���йؼ��㣻��ɫ��ѧ��Ӧ��

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�