��Ŀ����
 ��δ�ʯ���л�ø��������ȼ��һֱ�ǻ�ѧ��̽���Ŀ��⣬��ʯ�ͷ���õ������ͽ����ѻ����Ի�ø��������ȼ�ͣ�
��δ�ʯ���л�ø��������ȼ��һֱ�ǻ�ѧ��̽���Ŀ��⣬��ʯ�ͷ���õ������ͽ����ѻ����Ի�ø��������ȼ�ͣ�����1��ʯ���Ǻ���20��30��̼ԭ�ӵ������Ļ��������³ʹ�̬��
����2��ʯ�ʹ��ѻ���ͨ��ʹ��Al2O3��������
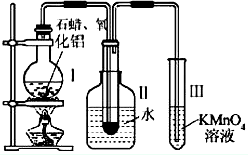
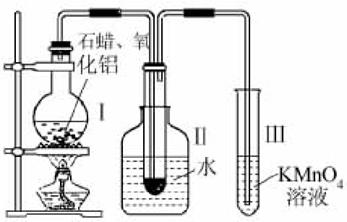
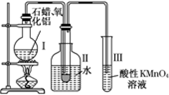
ij�о���ѧϰС����ʵ������ģ��ʯ�͵Ĵ��ѻ���װ����ͼ��ʵ������пɹ۲쵽��ƿ���й���ʯ�����ۻ����Թܢ���������Һ�����ᣬ�Թܢ������Ը��������Һ��ɫ��ʵ������Թܢ���Һ����ζ���������͵���ζ��
��1����װ���������ӵ�˳����ѭԭ��Ϊ
�������ϣ���������
�������ϣ���������
��Ϊ��֤ʵ��ɹ���ʵ��ǰ������еIJ��������װ�õ�������
���װ�õ�������
��װ���нϳ����ܵ���������������������
��������������
����2���Թܢ�������Һ������˵��
�ѻ������˺�5��̼ԭ�����ϵ���
�ѻ������˺�5��̼ԭ�����ϵ���
����3���Թܢ�����Һ��ɫ˵���ѻ�������̼ԭ����С��5��ϩ��
�ѻ�������̼ԭ����С��5��ϩ��
����4���ܷ����Թܢ��е�Һ����ȡ��ˮ�е��壬
����
����
���������ѻ���������ϩ���������巢���ӳɷ�Ӧ��
�ѻ���������ϩ���������巢���ӳɷ�Ӧ��
������ȡ��
������ȡ��
����5��д����ʮ���ѻ��õ������ϩ�Ļ�ѧ����ʽ
C20H42
C10H22+C10H20
| ||
| �� |
C20H42
C10H22+C10H20
| ||
| �� |
��6��ʯ���ѻ�����Ҫ������
�����ʯ�Ͳ�Ʒ������ȼ���ر������͵IJ���������
�����ʯ�Ͳ�Ʒ������ȼ���ر������͵IJ���������
����������1������������ȷ�����ӵ�ԭ�������װ��ҩƷ֮ǰҪ���װ�õ������ԣ�����ʯ�ͷ���ҵ�ķ�����ԭ����װ���нϳ����ܵ�����Ϊ�������������壻
��2�����ݴ��ѻ�ԭ���������º�5��̼ԭ�����ϵ�����Һ�������
��3����������KMnO4��Һ��ɫ�����³�ѹ�º�5��̼ԭ�����µ��������������
��4�������ѻ���������ϩ�����ɣ������巢���ӳɷ�Ӧ������
��5�����ݶ�ʮ����Al2O3�������������ѻ��õ������ϩд����Ӧ�ķ���ʽ��
��6��ʯ���ѻ������ʯ�Ͳ�Ʒ������ȼ���ر������͵IJ�����������
��2�����ݴ��ѻ�ԭ���������º�5��̼ԭ�����ϵ�����Һ�������
��3����������KMnO4��Һ��ɫ�����³�ѹ�º�5��̼ԭ�����µ��������������
��4�������ѻ���������ϩ�����ɣ������巢���ӳɷ�Ӧ������
��5�����ݶ�ʮ����Al2O3�������������ѻ��õ������ϩд����Ӧ�ķ���ʽ��
��6��ʯ���ѻ������ʯ�Ͳ�Ʒ������ȼ���ر������͵IJ�����������
����⣺��1���������ӵ�˳��Ӧ��ѭ�������ϡ������ҵ�ԭ��װ��ҩƷ֮ǰҪ���װ�õ������ԣ�����ʯ�ͷ���ҵ�ķ�����ԭ���������˳����ܣ������ó������⣬����������������ã�
�ʴ�Ϊ���������ϣ��������ң����װ�õ������ԣ��������������壻
��2�����ݴ��ѻ�ԭ�������з�Ӧ���п��ܷ�����a��C20H42
C10H20+C10H22��b��C10H22
C5H12+C5H10��c��C5H12
C2H4+C3H8��d��C5H12
CH4+C4H8���Թܢ�����Һ�����ɣ�˵�������˺�5��̼ԭ�����ϵ�������Ϊ���³�ѹ�£���5��̼ԭ�����ϵ�����Һ�壬
�ʴ�Ϊ���ѻ������˺�5��̼ԭ�����ϵ�����
��3���Թܢ�������KMnO4��Һ��ɫ˵��������������ԭ��Ӧ�������˳��³�ѹ�³���̬�ĺ�5��̼ԭ�����µ�ϩ����
�ʴ�Ϊ���ѻ�������̼ԭ����С��5��ϩ����
��4�������ѻ���������ϩ�����ɣ������巢���ӳɷ�Ӧ�����Բ������Թܢ��е�Һ����ȡ��ˮ�е��壬
�ʴ�Ϊ�����ܣ����ѻ���������ϩ���������巢���ӳɷ�Ӧ��������ȡ�壻
��5����ʮ����Al2O3�������������ѻ��õ������ϩ����Ӧ�Ļ�ѧ����ʽΪ��C20H42
C10H22+C10H20��
�ʴ�Ϊ��C20H42
C10H22+C10H20��
��6�������ϱ仯���Կ�����ʯ���ѻ�����ҪĿ����Ϊ�˻�ø��������ȼ�ͣ��ر���������͵IJ�����������
�ʴ�Ϊ�������ʯ�Ͳ�Ʒ������ȼ���ر������͵IJ�����������
�ʴ�Ϊ���������ϣ��������ң����װ�õ������ԣ��������������壻
��2�����ݴ��ѻ�ԭ�������з�Ӧ���п��ܷ�����a��C20H42
| Al2O3 |
| �� |
| ||
| �� |
| ||
| �� |
| ||
�ʴ�Ϊ���ѻ������˺�5��̼ԭ�����ϵ�����
��3���Թܢ�������KMnO4��Һ��ɫ˵��������������ԭ��Ӧ�������˳��³�ѹ�³���̬�ĺ�5��̼ԭ�����µ�ϩ����
�ʴ�Ϊ���ѻ�������̼ԭ����С��5��ϩ����
��4�������ѻ���������ϩ�����ɣ������巢���ӳɷ�Ӧ�����Բ������Թܢ��е�Һ����ȡ��ˮ�е��壬
�ʴ�Ϊ�����ܣ����ѻ���������ϩ���������巢���ӳɷ�Ӧ��������ȡ�壻
��5����ʮ����Al2O3�������������ѻ��õ������ϩ����Ӧ�Ļ�ѧ����ʽΪ��C20H42
| ||
| �� |
�ʴ�Ϊ��C20H42
| ||
| �� |
��6�������ϱ仯���Կ�����ʯ���ѻ�����ҪĿ����Ϊ�˻�ø��������ȼ�ͣ��ر���������͵IJ�����������
�ʴ�Ϊ�������ʯ�Ͳ�Ʒ������ȼ���ر������͵IJ�����������
���������⿼����ʯ�͵ķ�����Ŀ�ѶȲ�����ɱ���ؼ��Ǻ�������������Ϣ������ʯ�͵ķ���ԭ����ɣ���ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ