��Ŀ����
�ٻ�2005��ŵ������ѧ������λ��ѧ����ϩ�����ֽⷴӦ�о�����ȡ���������ɾ͡�ϩ���Ľ��渴�ֽⷴӦ��������Ϊ˫�����ѣ���ȫ�п�������λ���ӡ�
�磺CH2=CHR1+CH2=CHR2 CH2=CH2+R1CH=CHR2
CH2=CH2+R1CH=CHR2
��֪����R-CH2-CH=CH2+Cl2 R-CHCl-CH=CH2+HCl
R-CHCl-CH=CH2+HCl
��F����֬ˮ�����֮һ������ˮ������Ȼ��ܣ�K��һ�����ϣ�I�Ļ�ѧʽΪC9H14O6��
�ۻ�����(C5H10)�Ľṹ��ʽ��ʾΪ ����ת����ϵ��ͼ��ʾ
����ת����ϵ��ͼ��ʾ
�磺CH2=CHR1+CH2=CHR2
 CH2=CH2+R1CH=CHR2
CH2=CH2+R1CH=CHR2 ��֪����R-CH2-CH=CH2+Cl2
 R-CHCl-CH=CH2+HCl
R-CHCl-CH=CH2+HCl ��F����֬ˮ�����֮һ������ˮ������Ȼ��ܣ�K��һ�����ϣ�I�Ļ�ѧʽΪC9H14O6��
�ۻ�����(C5H10)�Ľṹ��ʽ��ʾΪ
 ����ת����ϵ��ͼ��ʾ
����ת����ϵ��ͼ��ʾ
��ش���������
(1)C4H8���ڻ�������ͬ���칹����_______�֣�
(2)д���������ʵĽṹ��ʽ��A_________��I_________��
(3)д�����з�Ӧ�ķ�Ӧ���ͣ�����ţ��� H+F��I_____��E��F____��
a���ӳ� b��ȡ�� c������ d����ȥ
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��
A+B��C_______________________C��D________________________��
(5)K��������____��K����____������Ρ������Ρ��ṹ����
(6)��ѧʽΪC4H8������ϩ��ͨ��ϩ�����ֽⷴӦ�����ɵ�C6H12��___ �ֽṹ���Ҵ���B��������____��
(1)C4H8���ڻ�������ͬ���칹����_______�֣�
(2)д���������ʵĽṹ��ʽ��A_________��I_________��
(3)д�����з�Ӧ�ķ�Ӧ���ͣ�����ţ��� H+F��I_____��E��F____��
a���ӳ� b��ȡ�� c������ d����ȥ
(4)д�����з�Ӧ�Ļ�ѧ����ʽ��
A+B��C_______________________C��D________________________��
(5)K��������____��K����____������Ρ������Ρ��ṹ����
(6)��ѧʽΪC4H8������ϩ��ͨ��ϩ�����ֽⷴӦ�����ɵ�C6H12��___ �ֽṹ���Ҵ���B��������____��
(1)2
(2)CH3-CH=CH-CH3��
(3)bc��b
(2)CH3-CH=CH-CH3��

(3)bc��b
(4)CH3-CH=CH-CH3+CH2=CH2 2CH3-CH=CH2
2CH3-CH=CH2
 2CH3-CH=CH2
2CH3-CH=CH2 CH3-CH=CH2+Cl2

(5) ������
������
(6)3��ŨH2SO4��170��
 ������
������(6)3��ŨH2SO4��170��

��ϰ��ϵ�д�
 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�����Ŀ
 CH2=CH2+R1CH=CHR2
CH2=CH2+R1CH=CHR2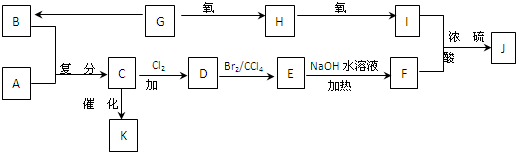
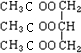
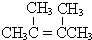
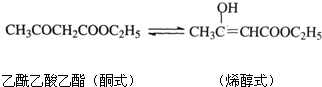
 CH2=CH2 + R1CH=CHR2
CH2=CH2 + R1CH=CHR2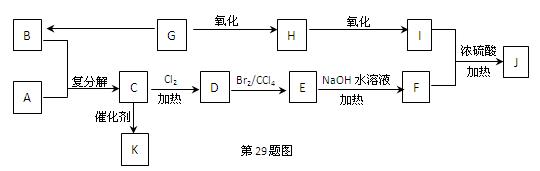

 CH2=CH2 + R1CH=CHR2
CH2=CH2 + R1CH=CHR2
