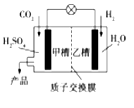
��Ŀ����
����Ŀ��36.5 g HCl�ܽ���1 Lˮ��(ˮ���ܶȽ���Ϊ1 g��mL��1)������Һ���ܶ�Ϊ�� g��mL��1����������Ϊw�����ʵ���Ũ��Ϊc mol��L��1��NA��ʾ�����ӵ���������ֵ����������������ȷ����
A��������Һ�����ʵ���Ũ��Ϊ1 mol.L��1
B��������Һ�к���NA��HCl����
C��36.5 g HCl����ռ�е����Ϊ22.4 L
D��������Һ������������w��![]()
���𰸡�D
��������
���������A��36.5gHCl�ܽ���1Lˮ��(ˮ���ܶȽ���Ϊ1g/mL)��������Һ����Ϊ�Ȼ��⣬���ʵ���Ũ��c=![]() =
=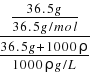 =
=![]() mol/L����A����B���Ȼ�������ˮ�γ�������Һ��ǿ����ʣ���Һ�����Ȼ�����ӣ���B����C��36.5gHCl�������ʵ���Ϊ1mol����״����ռ�е����Ϊ22.4L��û˵��״���£���C������D��������Һ��������������=
mol/L����A����B���Ȼ�������ˮ�γ�������Һ��ǿ����ʣ���Һ�����Ȼ�����ӣ���B����C��36.5gHCl�������ʵ���Ϊ1mol����״����ռ�е����Ϊ22.4L��û˵��״���£���C������D��������Һ��������������=![]() =
=![]() ����D��ȷ����ѡD��
����D��ȷ����ѡD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��(1)����ÿ��ֱ�����������������п������һ�����ʵ�����ʽ������(����)___________
�� | �� | �� | �� | �� |
�������������� | ������� | ��Һ�������ٷֱ�Ũ�� | ��״���������Ħ����� | �DZ�״����ij���ʵ����� |
�����ӵ����� | �����ܶ� | ��Һ��� | ��״������������ | ���ʵ�Ħ������ |
(2)�ڱ�״���£���6.72LCH4�� ��3.01��1023��HCl���ӣ� ��13.6gH2S�� ��0.2molNH3�����ж������������������ȷ����(��д����)___________
A��������>��>��>�� B���ܶȢ�>��>��>�� C�������>��>��>�� D����ԭ������>��>��>��