��Ŀ����
��8�֣���֪��
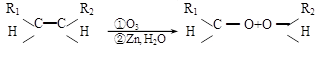
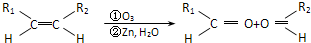
��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________������ţ���
![]() �١�CH3 �� �ۡ�CH�TCH2
�١�CH3 �� �ۡ�CH�TCH2
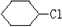
��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
�ṹ��������E�����к�����������û��֧����
��д��A![]() B�Ļ�ѧ����ʽ��
B�Ļ�ѧ����ʽ��
��E�Ľṹ��ʽΪ��
��д��D��ͬʱ��������������ͬ���칹��Ľṹ��ʽ�� a.���ڶ�Ԫ���� b.��������4��̼ԭ�ӣ�
��ÿ��1�֣���8�֣���1��ȩ�����٢� ��2���ڢ�
����:��

��8�֣���֪��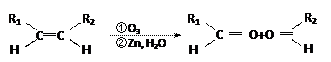
��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________�� ����ţ���
����ţ��� �١�CH3 �� �ۡ�CH�TCH2
�١�CH3 �� �ۡ�CH�TCH2
��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
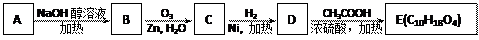 |
�ṹ��������E�����к�����������û��֧����
���A
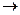 B�Ļ�ѧ����ʽ��
B�Ļ�ѧ����ʽ�� ��E�Ľṹ��ʽΪ��
��д��D��ͬʱ��������������ͬ���칹��Ľṹ��ʽ�� a.���ڶ�Ԫ���� b.��������4��̼ԭ�ӣ�
��8�֣���֪��
��1���÷�Ӧ���������к��еĹ����ŵ�������__________����������һ���������ܷ���_______������ţ���
��������Ӧ �ڻ�ԭ��Ӧ ��������Ӧ
��2����֪HCHO����������ԭ�Ӷ���ͬһƽ���ڣ���ҪʹR1CHO����������ԭ�ӿ��ܶ���ͬһƽ���ڣ�R1������________������ţ���
 �١�CH3 �� �ۡ�CH�TCH2
�١�CH3 �� �ۡ�CH�TCH2
��3��ij�ȴ���A�ķ���ʽΪC6H11Cl�������Է�������ת����
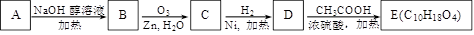 |
�ṹ��������E�����к�����������û��֧����
��д��A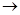 B�Ļ�ѧ����ʽ��
B�Ļ�ѧ����ʽ��
��E�Ľṹ��ʽΪ��
��д��D��ͬʱ��������������ͬ���칹��Ľṹ��ʽ�� a.���ڶ�Ԫ���� b.��������4��̼ԭ�ӣ�
 ��
��


 ��-CH�TCH2
��-CH�TCH2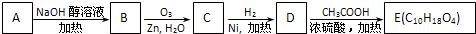
 +NaOH
+NaOH +NaCl+H2O
+NaCl+H2O