��Ŀ����
��2010?�Ͼ���ģ�������������ƶ���������Ⱦ����Ҫ�����ֶΣ�
��1�����ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��
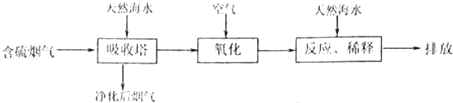
ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ1��ʾ��
�ٸ���ͼʾʵ������Ϊ�����һ��Ũ�Ⱥ���������SO2������Ч�ʣ����д�ʩ��ȷ����
A������ͨ�뺬���������¶ȣ�B����Сͨ�뺬������������
C��������Ȼ��ˮ�Ľ�������D������Ȼ��ˮ�м�����ʯ��
����Ȼ��ˮ�����˺��������������H2SO3��ʹ�ÿ����е���������������д���÷�Ӧ�����ӷ���ʽ
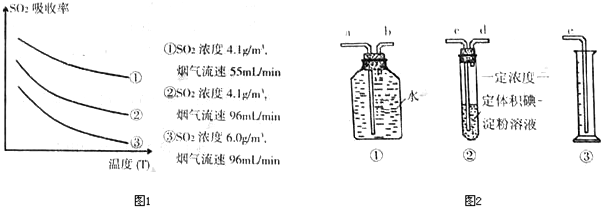
�۸�С�����ͼ2װ����ʵ���Ҳⶨ������SO2���������������ʵ���ڱ�״���½��У�������װ����װ���ӵ�˳���ǣ�ԭ������
A������KMnO4��Һ��B��NaOH��Һ��C����ˮ��D����ˮ
��2��ʯ��ʯ-ʯ��ʪ�����������ռ����Ĺ���ԭ���������еĶ��������뽬Һ�е�̼����Լ�����Ŀ�����Ӧ����ʯ�ࣨCaSO4?2H2O����д���÷�Ӧ�Ļ�ѧ����ʽ��
��1�����ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��

ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ1��ʾ��

�ٸ���ͼʾʵ������Ϊ�����һ��Ũ�Ⱥ���������SO2������Ч�ʣ����д�ʩ��ȷ����
ABD
ABD
��A������ͨ�뺬���������¶ȣ�B����Сͨ�뺬������������
C��������Ȼ��ˮ�Ľ�������D������Ȼ��ˮ�м�����ʯ��
����Ȼ��ˮ�����˺��������������H2SO3��ʹ�ÿ����е���������������д���÷�Ӧ�����ӷ���ʽ
2H2SO3+O2=2H2SO4
2H2SO3+O2=2H2SO4
���۸�С�����ͼ2װ����ʵ���Ҳⶨ������SO2���������������ʵ���ڱ�״���½��У�������װ����װ���ӵ�˳���ǣ�ԭ������
cdbae
cdbae
����a��b��c��d��e���������Լ��У�Ũ�ȡ����һ�������������������Թ��еĵ�-������Һ����AC
AC
�����ţ���A������KMnO4��Һ��B��NaOH��Һ��C����ˮ��D����ˮ
��2��ʯ��ʯ-ʯ��ʪ�����������ռ����Ĺ���ԭ���������еĶ��������뽬Һ�е�̼����Լ�����Ŀ�����Ӧ����ʯ�ࣨCaSO4?2H2O����д���÷�Ӧ�Ļ�ѧ����ʽ��
2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
��ij�糧��ú300�֣�ú�к�����������Ϊ2.5%������ȼ��ʱú�е���ȫ��ת���ɶ���������ʯ��ʪ��������������96%����ת��Ϊʯ�࣬�������ʯ��38.7
38.7
�֣���������1��������������SO2������Ч�����¶ȵ����߶����ͣ����¶Ⱥ�Ũ����ͬʱ��SO2������Ч�����������ٵ���������ͣ����¶Ⱥ�����������ͬʱ��SO2������Ч����SO2Ũ�ȵ���������ͣ�ͬʱ������ʯ������ˮ��Ӧ���ɼ������SO2��Ӧ��
�ڸ���ʹ�ÿ����е�������H2SO3������
�۸���ʵ���ԭ�����������ⶨģ��������SO2������������ɸ��ݶ�����������Ļ�ԭ�ԣ�����������Ե����Ը�����ػ���ˮ��Ӧ��ͨ����ɫ�ı仯�жϣ�
��2������������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O�����ݹ�ϵʽS��SO2��CaSO4?2H2O�����㣻
�ڸ���ʹ�ÿ����е�������H2SO3������
�۸���ʵ���ԭ�����������ⶨģ��������SO2������������ɸ��ݶ�����������Ļ�ԭ�ԣ�����������Ե����Ը�����ػ���ˮ��Ӧ��ͨ����ɫ�ı仯�жϣ�
��2������������̼��Ʒ�Ӧ������������������̼�����������ˮ���ڵ������±�������������CaSO4?2H2O�����ݹ�ϵʽS��SO2��CaSO4?2H2O�����㣻
����⣺��1��������������SO2������Ч�����¶ȵ����߶����ͣ����¶Ⱥ�Ũ����ͬʱ��SO2������Ч�����������ٵ���������ͣ����¶Ⱥ�����������ͬʱ��SO2������Ч����SO2Ũ�ȵ���������ͣ�ͬʱ������ʯ������ˮ��Ӧ���ɼ������SO2��Ӧ������Ϊ�����һ��Ũ�Ⱥ���������SO2������Ч�ʣ���ͨ������ͨ�뺬���������¶ȣ���Сͨ�뺬�������������Լ�����Ȼ��ˮ�м�����ʯ�ң��ʴ�Ϊ��ABD��
���ɿ����е�������H2SO3����Ϊ���ᣬ�÷�ӦΪ2H2SO3+O2=2H2SO4��
�ʴ�Ϊ��2H2SO3+O2=2H2SO4��
�۸�ʵ��IJⶨԭ��Ϊ������ͨ��һ��Ũ��һ������ĵ�-������Һ�����е�SO2��ⷴӦ����ǡ�÷�Ӧʱ����Һ����ɫ��ʧ�����ݵ�����ʵ����������SO2�����ʵ��������µ�����������ƿ����ˮѹ����Ͳ������Ͳ�е�ˮ���������ȷ��ʣ������������������SO2����������ⶨģ��������SO2������������ɸ��ݶ�����������Ļ�ԭ�ԣ�����������Ե����Ը�����ػ���ˮ��Ӧ��ͨ����ɫ�ı仯�жϣ�ѡ����AC���ϣ�������ɫ���Ҷ����������ԣ������������Ӧ����ѡ��cdbae��AC��
��2������������̼��Ʒ�Ӧ������������������̼����Ӧ����ʽΪ��SO2+CaCO3=CaSO3+CO2�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ����ʽΪ��2CaSO3+O2+4H2O=2��CaSO4?2H2O�����ܷ�ӦΪ��2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
�ʴ�Ϊ��SO2+CaCO3=CaSO3+CO2��2CaSO3+O2+4H2O=2��CaSO4?2H2O����
S��SO2 ��CaSO4?2H2O
32 172
300t��2.5%��96% m
=
�����m=38.7t��
�ʴ�Ϊ��38.7��
���ɿ����е�������H2SO3����Ϊ���ᣬ�÷�ӦΪ2H2SO3+O2=2H2SO4��
�ʴ�Ϊ��2H2SO3+O2=2H2SO4��
�۸�ʵ��IJⶨԭ��Ϊ������ͨ��һ��Ũ��һ������ĵ�-������Һ�����е�SO2��ⷴӦ����ǡ�÷�Ӧʱ����Һ����ɫ��ʧ�����ݵ�����ʵ����������SO2�����ʵ��������µ�����������ƿ����ˮѹ����Ͳ������Ͳ�е�ˮ���������ȷ��ʣ������������������SO2����������ⶨģ��������SO2������������ɸ��ݶ�����������Ļ�ԭ�ԣ�����������Ե����Ը�����ػ���ˮ��Ӧ��ͨ����ɫ�ı仯�жϣ�ѡ����AC���ϣ�������ɫ���Ҷ����������ԣ������������Ӧ����ѡ��cdbae��AC��
��2������������̼��Ʒ�Ӧ������������������̼����Ӧ����ʽΪ��SO2+CaCO3=CaSO3+CO2�����������ˮ���ڵ������±�������������CaSO4?2H2O����Ӧ����ʽΪ��2CaSO3+O2+4H2O=2��CaSO4?2H2O�����ܷ�ӦΪ��2CaCO3+2SO2+O2+4H2O�T2��CaSO4?2H2O��+2CO2��
�ʴ�Ϊ��SO2+CaCO3=CaSO3+CO2��2CaSO3+O2+4H2O=2��CaSO4?2H2O����
S��SO2 ��CaSO4?2H2O
32 172
300t��2.5%��96% m
| 32 |
| 300t��2.5%��96% |
| 172 |
| m |
�ʴ�Ϊ��38.7��
���������⿼����Ⱦ�Ĵ�����������ѧ֪ʶ���ϰ���е���Ϣ���ɽ��ϰ����ͼ�������ǽ����Ĺؼ����ϺõĿ���ѧ���������⡢��������������

��ϰ��ϵ�д�
�����Ŀ