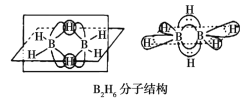
��Ŀ����
����Ŀ����NAΪ����٤��������ֵ������˵����ȷ����
A.5NH4NO3 ![]() 2HNO3��4N2����9H2O��Ӧ�У�����28 g N2��ת�Ƶĵ�����ĿΪ3.75NA
2HNO3��4N2����9H2O��Ӧ�У�����28 g N2��ת�Ƶĵ�����ĿΪ3.75NA
B.�����£�1 L pH��13��NaOH��Һ�У���ˮ�����OH�� ��ĿΪ0.1NA
C.����ȼ�ϵ����������22.4 L����״��������ʱ����·��ͨ���ĵ�����ĿΪ2NA
D.�����£�0.2 mol Fe������ˮ������Ӧ�����ɵ�H2������ĿΪ0.3 NA
���𰸡�A
��������
A�����ݷ�Ӧ����ʽ������4molN2ת�Ƶ���15mol��������28gN2ʱת�Ƶ��ӵ����ʵ���Ϊ![]() mol=3.75mol����A��ȷ��
mol=3.75mol����A��ȷ��
B��������Һ�м���ˮ�����OH�����ʵ�������Ҫ������Һ��H������Ϊˮ�������H����OH�����ʵ�����ȣ������n(H��)=1��10��13mol=n(OH��)����B����
C������ȼ�ϵ�أ�������������1molO2�μӷ�Ӧת��4mole������C����
D��3Fe��4H2O(g)![]() Fe3O4��4H2�����ݷ�Ӧ����ʽ������0.2mol��ʱ���������������ʵ���Ϊ
Fe3O4��4H2�����ݷ�Ӧ����ʽ������0.2mol��ʱ���������������ʵ���Ϊ![]() mol����D����
mol����D����
�ʴ�ѡA��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�����Ŀ���±����и��������У�����֮��ͨ��һ����Ӧ����ʵ������ͼ��ʾת������
ѡ�� | X | Y | Z |
A | Na | NaOH | NaCl |
B | Si | SiO2 | Na2SiO3 |
C | Cl2 | HClO | NaClO |
D | NO | NO2 | HNO3 |
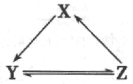
A. A B. B C. C D. D