��Ŀ����
��8�֣���һ������̼���⡢������Ԫ����ɵ��л�������A��������4.8g������ϵ�ȼ���ٽ����ɵ���������ͨ����ʢ��Ũ�����ϴ��ƿ�ڱ���ʯ��ˮ����ÿ��װ���еķ�Ӧ��������������ⶨ��������3.60g ��������8.80g��A�������ܶ�����ͬ������H2��38�����ֲ�֪���л��������̼������Һ��Ӧ����������Ʒ�Ӧ���Ҿ����������ɣ����õ�����������ͬ�����������ͬ��������л���ķ���ʽ����д������ܵĽṹ��ʽ����д����Ҫ�ļ�����̣�
C2H4O3,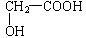
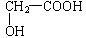
���⿼���л���ȼ�յ��йؼ�����йؽṹ��ʽ���ƶϡ������л���ȼ�յ�ͨʽ������غ��ϵ�ɵó�����ʽ��Ȼ�����ݻ�ѧ���ʿɵó���ṹ��ʽ��
��1��Ũ������ˮ�����ˮ��������3.60g�����ʵ���Ϊ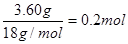 ��������ԭ�ӵ�������0.4g����ԭ�ӵ�������0.2mol��16g/mol=3.2g��ʯ��ˮ���յ���CO2������CO2��������8.8g�������ʵ�����
��������ԭ�ӵ�������0.4g����ԭ�ӵ�������0.2mol��16g/mol=3.2g��ʯ��ˮ���յ���CO2������CO2��������8.8g�������ʵ�����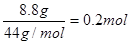 ������̼ԭ�ӵ�������0.2mol��12g/mol��2.4g��ԭ�ӵ�������8.8g��2.4g��6.4g��������ԭ���غ��֪���л����к��е���ԭ�ӵ�������3.2g��6.4g��4.8g��4.8g�������ʵ�����
������̼ԭ�ӵ�������0.2mol��12g/mol��2.4g��ԭ�ӵ�������8.8g��2.4g��6.4g��������ԭ���غ��֪���л����к��е���ԭ�ӵ�������3.2g��6.4g��4.8g��4.8g�������ʵ�����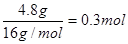 �������л�����C��H��O��ԭ�Ӹ���֮��Ϊ2�U4�U3�������ʽΪC2H4O3��
�������л�����C��H��O��ԭ�Ӹ���֮��Ϊ2�U4�U3�������ʽΪC2H4O3��
��2����ΪA�������ܶ�����ͬ������H2��38��������A����Է���������38��2��76�����Ը������ʽ����Է���������֪���л���ķ���ʽΪC2H4O3��
��3��A������̼������Һ��Ӧ����������Ʒ�Ӧ���Ҿ����������ɣ�����A��һ�������Ȼ���Ҳ���ܺ����ǻ�������ֻ��3����ԭ�ӣ�����һ�������ǻ������ɵ�������CO2��H2�������ṹ��ʽΪ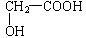 ��
��
��1��Ũ������ˮ�����ˮ��������3.60g�����ʵ���Ϊ
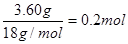 ��������ԭ�ӵ�������0.4g����ԭ�ӵ�������0.2mol��16g/mol=3.2g��ʯ��ˮ���յ���CO2������CO2��������8.8g�������ʵ�����
��������ԭ�ӵ�������0.4g����ԭ�ӵ�������0.2mol��16g/mol=3.2g��ʯ��ˮ���յ���CO2������CO2��������8.8g�������ʵ�����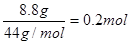 ������̼ԭ�ӵ�������0.2mol��12g/mol��2.4g��ԭ�ӵ�������8.8g��2.4g��6.4g��������ԭ���غ��֪���л����к��е���ԭ�ӵ�������3.2g��6.4g��4.8g��4.8g�������ʵ�����
������̼ԭ�ӵ�������0.2mol��12g/mol��2.4g��ԭ�ӵ�������8.8g��2.4g��6.4g��������ԭ���غ��֪���л����к��е���ԭ�ӵ�������3.2g��6.4g��4.8g��4.8g�������ʵ�����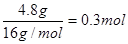 �������л�����C��H��O��ԭ�Ӹ���֮��Ϊ2�U4�U3�������ʽΪC2H4O3��
�������л�����C��H��O��ԭ�Ӹ���֮��Ϊ2�U4�U3�������ʽΪC2H4O3����2����ΪA�������ܶ�����ͬ������H2��38��������A����Է���������38��2��76�����Ը������ʽ����Է���������֪���л���ķ���ʽΪC2H4O3��
��3��A������̼������Һ��Ӧ����������Ʒ�Ӧ���Ҿ����������ɣ�����A��һ�������Ȼ���Ҳ���ܺ����ǻ�������ֻ��3����ԭ�ӣ�����һ�������ǻ������ɵ�������CO2��H2�������ṹ��ʽΪ
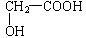 ��
��
��ϰ��ϵ�д�
�����Ŀ
 ��ʾ�ķ���ʽ �������� ��
��ʾ�ķ���ʽ �������� �� �к��еĹ����ŵ�����Ϊ ��
�к��еĹ����ŵ�����Ϊ �� ȡ����Ӧ
ȡ����Ӧ BrCH2CH2Br�ӳɷ�Ӧ
BrCH2CH2Br�ӳɷ�Ӧ ������Ӧ
������Ӧ