��Ŀ����
25 ��ʱ���������ʵĵ���ƽ�ⳣ�������ʾ��
��ѧʽ | CH3COOH | H2C2O4 | H2S |
����ƽ�ⳣ�� | 1.8��10-5 | Ka1=5.4��10-2 Ka2=5.4��10-5 | Ka1=1.3��10-7 Ka2=7.1��10-15 |
��ش��������⣺
��1��H2S��һ�����볣������ʽΪKa1=____________________
��2��CH3COOH��H2C2O4��H2S��������ǿ������˳��________________________
��3��H2C2O4��������KOH��Һ��Ӧ�Ļ�ѧ����ʽ��_______________________
��4��NaHS��Һ��NaHC2O4��Һ��Ӧ�����ӷ���ʽ��_______________________
��5��H��Ũ����ͬ�������������ҺA�����ᣩ��B��CH3COOH���ֱ���п�۷�Ӧ����������һ����Һ�д���п���ų�������������ͬ��������˵����ȷ����__________����д��ţ���
�ٷ�Ӧ����Ҫ��ʱ��B��A
�ڿ�ʼ��Ӧʱ������A��B
�۲μӷ�Ӧ��п�����ʵ���A=B
�ܷ�Ӧ���̵�ƽ������B��A
��B����пʣ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�ú�����г����о���ͬ�¶���ƽ�ⳣ����Ͷ�ϱȵ����⡣
��֪��CO(g)+H2O(g) H2(g)+CO2(g)ƽ�ⳣ��K���¶ȵı仯���±���
H2(g)+CO2(g)ƽ�ⳣ��K���¶ȵı仯���±���
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
�ش���������
(1)�÷�Ӧ��ƽ�ⳣ������ʽK= ����H 0(�����������������=��)
(2)��֪��һ���¶��£�C(s)+CO2(g) 2CO(g)ƽ�ⳣ��K1
2CO(g)ƽ�ⳣ��K1
C(s)+H2O(g) H2(g)+CO(g)ƽ�ⳣ��K2��
H2(g)+CO(g)ƽ�ⳣ��K2��
��K��K1��K2��֮��Ĺ�ϵ�� ��
(3)800��ʱ����һ��10L�ĺ��ݷ�Ӧ���г���0.40molCO��1.60molˮ��������һ��ʱ ���Ӧ�ﵽƽ�⣬��ʱCO��ת����Ϊ �������������������䣬��ƽ����ϵ����ͨ��0.10molCO��0.40mol CO2����ʱv�� v�� (���������=������)��
��ס��ҡ��������ܱ������г���һ������A��B��������Ӧ��A��g����xB��g�� 2C��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����±�����ͼ��ʾ��
2C��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����±�����ͼ��ʾ��

���� | �� | �� | �� |
�ݻ� | 0.5 L | 0.5 L | 1.0 L |
�¶�/�� | T1 | T2 | T2 |
��Ӧ�� ��ʼ�� | 1.5 mol A 0.5 mol B | 1.5 mol A 0.5 mol B | 6.0 mol A 2.0 mol B |
����˵����ȷ���ǣ� ��
A.ƽ�������������ٳ���0.5 mol A��A��B��ת���ʾ�����
B.�ﵽƽ��ʱ��Ӧ���յ�������Q��> 2Q��
C.��ƽ��ʱ�����¶Ȳ��䣬�ı��������ƽ�ⲻ�ƶ�
D��T1�棬��ʼʱ�������г���0.5 mol A��1.5 mol B��ƽ��ʱA��ת����Ϊ25%


 ��1molˮ���ӵ�
��1molˮ���ӵ� �������
������� �� C������������
�� C������������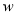 �� D��Ħ��������M��
�� D��Ħ��������M�� =2X2����5Z2��8H2O
=2X2����5Z2��8H2O ����˳����XO
����˳����XO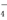 ��Z2��R2��M3��
��Z2��R2��M3�� ��
��