��Ŀ����
��11�֣�
2-1 ����2,4-���ͪ��������Mn3+�γɵĵ����������Ľṹʽ�������� ��ʾ����
��ʾ����
2-2 ��֪�������Ĵž�Ϊ4.9�������ӣ��������Mn��δ�ɶԵ�����Ϊ ��
2-3 �ش𣺸û������������ԣ�Ϊʲô��
2-4 ����2,4���ͪ�����ӵĽṹ��ʽ��������ȷ�乲��֣���д����������м��ı�ʾ���š�
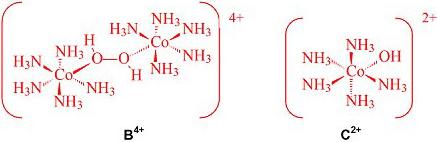
2-5 �Ȼ�ɫ���������A�����������Ȼ���������(��)���ǽ����Ȼ��ܡ�Ũ��ˮ���Ȼ�狀��������ϣ��Ի���̿Ϊ�����ϳɵġ������о����֣���Ӧ���������ȵõ�Co(NH3)62+���ӣ����������ȡ����Ӧ���õ���������Ϊ�ż���˫������B4+�����ŷ����ż����ѣ�ͬʱ2������ԭ�ӷֱ�1�����Ӵ��ݵ����Ѻ���������ϣ��õ�2��C2+���ӣ����C2+�����ڻ���̿�����Ϸ�������ȡ����Ӧ�����������ӽ���γɹ��������A��д���ϳ������A���ܷ�Ӧ����ʽ������B4+��C2+���ӵĽṹʽ��
�ܷ�Ӧ����ʽ��
B4+��C2+���ӵĽṹʽ��
2-1 ����2,4-���ͪ��������Mn3+�γɵĵ����������Ľṹʽ��������
 ��ʾ����
��ʾ����2-2 ��֪�������Ĵž�Ϊ4.9�������ӣ��������Mn��δ�ɶԵ�����Ϊ ��
2-3 �ش𣺸û������������ԣ�Ϊʲô��
2-4 ����2,4���ͪ�����ӵĽṹ��ʽ��������ȷ�乲��֣���д����������м��ı�ʾ���š�
2-5 �Ȼ�ɫ���������A�����������Ȼ���������(��)���ǽ����Ȼ��ܡ�Ũ��ˮ���Ȼ�狀��������ϣ��Ի���̿Ϊ�����ϳɵġ������о����֣���Ӧ���������ȵõ�Co(NH3)62+���ӣ����������ȡ����Ӧ���õ���������Ϊ�ż���˫������B4+�����ŷ����ż����ѣ�ͬʱ2������ԭ�ӷֱ�1�����Ӵ��ݵ����Ѻ���������ϣ��õ�2��C2+���ӣ����C2+�����ڻ���̿�����Ϸ�������ȡ����Ӧ�����������ӽ���γɹ��������A��д���ϳ������A���ܷ�Ӧ����ʽ������B4+��C2+���ӵĽṹʽ��
�ܷ�Ӧ����ʽ��
B4+��C2+���ӵĽṹʽ��
��11�֣�2-1

2-2 4
2-3 �С��û�����ֻ����ת�ᣨ��1��Գ�Ԫ�أ���
2-4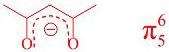
2-5 2CoCl2 + 10NH3 + 2NH4Cl + H2O2�� 2Co(NH3)6Cl3 + 2H2O

2-2 4
2-3 �С��û�����ֻ����ת�ᣨ��1��Գ�Ԫ�أ���
2-4

2-5 2CoCl2 + 10NH3 + 2NH4Cl + H2O2�� 2Co(NH3)6Cl3 + 2H2O

��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��д��������NaOH��Һ��Ӧ�����ӷ���ʽ�� ��
��д��������NaOH��Һ��Ӧ�����ӷ���ʽ�� �� ͬһ���塣X��A��B��C��D��E��F��G��Ϊ�����Ļ��������A��X��Ħ��������ͬ��A��G����ɫ��ӦΪ��ɫ����һ�������£��������ת����ϵ����ͼ����ش�
ͬһ���塣X��A��B��C��D��E��F��G��Ϊ�����Ļ��������A��X��Ħ��������ͬ��A��G����ɫ��ӦΪ��ɫ����һ�������£��������ת����ϵ����ͼ����ش�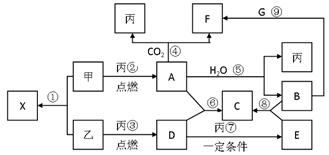
 Ӧ�У�������������ԭ��Ӧ���ǣ�����ţ�____________________��
Ӧ�У�������������ԭ��Ӧ���ǣ�����ţ�____________________�� ����Һ�е����ʼ������ʵ����ֱ�Ϊ ����ͬ���������Dͨ��100mL 2��5mo
����Һ�е����ʼ������ʵ����ֱ�Ϊ ����ͬ���������Dͨ��100mL 2��5mo l��L-1��B��Һ�У���ȫ���պ���Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ ��
l��L-1��B��Һ�У���ȫ���պ���Һ���������ӵ����ʵ���Ũ���ɴ�С��˳��Ϊ �� ���÷�Ӧ �����ǻ��ǣ�����-��ԭ��Ӧ
���÷�Ӧ �����ǻ��ǣ�����-��ԭ��Ӧ