��Ŀ����
�ڻ�ҩ���й��Ŵ��Ĵ���֮һ�����ı�ը��ӦΪ��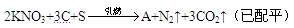
(1)�������г��˵����������̼�⣬����һ������A���ɣ���A�ĵ���ʽΪ ��A���� ���塣
(2)�ڻ�ҩ��λ��Ԫ�����ڱ������ڵ�Ԫ���� �֡�����һ��Ԫ�صĵ��ʿ�������ҩ���������������Ư��֯��ͷ���������Ԫ�������ڱ��е�λ���� ����Ԫ�ص�ԭ�Ӻ����� �ֲ�ͬ�˶�״̬�ĵ��ӡ�
(3)�ڻ�ҩ������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� (��Ԫ�ط��ű�ʾ)��
(4)������ʵ��˵���ڻ�ҩ��̼������Ԫ�طǽ��������ǿ������ ��
| A��ͬ����ͬŨ����ҺpH��Na2CO3��Na2SO4 | B�����ԣ�H2SO3��H2CO3 |
| C��CS2��̼Ԫ��Ϊ+4�ۣ���Ԫ��Ϊ-2�� | D���ֽ��¶ȣ�CH4��H2S |
(1)��2�֣� �����ӡ���ÿ��1�֣�
�����ӡ���ÿ��1�֣�
(2)��2�֣�4���������ڵ�VIA�壬16��������ȫ��2�֣���һ������1�֣�
(3)��2�֣�K>S>C>N>O
(4)��2�֣�A��C��һ��1�֣���һ����2�֣�
����

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д��ڻ�ҩ���й��Ŵ��Ĵ���֮һ�����ı�ը��ӦΪ��
2KNO3+3C+S  K2S+N2��+3a ����ƽ
K2S+N2��+3a ����ƽ
�ٻ�����ԭ�ӵĽṹʾ��ͼ ��
���������г����������⣬����һ������A���ɣ���A�Ļ�ѧʽΪ ��
�ۺڻ�ҩ��λ��Ԫ�����ڱ������ڵ�Ԫ���� �֣�����һ��Ԫ�ص������������Ư��ֽ����˿����ñ��Ȳ�������ɱ������������Ԫ�������ڱ��е�λ���� ��д����������ͨ��FeCl3��Һ��Ӧ�����ӷ���ʽ ��
��������ʵ��˵���ڻ�ҩ��̼������Ԫ�طǽ��������ǿ������ ��
| A��ͬ����ͬŨ����Һ��pH��H2CO3��H2SO4 | B���ȶ��ԣ�H2SO4��H2CO3 |
| C��CS2��̼Ԫ��Ϊ+4�ۣ���Ԫ��Ϊ-2�� | D���ܽ��ԣ�H2S��CH4 |
2��10��18 mol/L��CuCl2��Һ,��ͨ������˵������CuS�������ɣ�д�������������̣�������Һ���ʱ������仯���� ��
 K2S+N2��+3a ����ƽ
K2S+N2��+3a ����ƽ