��Ŀ����
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K+��NH4+��Cl����Mg2+��Fe3+��CO32����SO42������ÿ��ȡ100ml����ʵ�顣(��֪NH4+��OH ����Һ�пɷ������з�Ӧ NH4+ + OH
����Һ�пɷ������з�Ӧ NH4+ + OH ��NH3
��NH3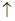 +H2O)
+H2O)
��1����һ�ݼ���AgNO3��Һ�г���������
��2���ڶ��ݼ�����NaOH����ȣ��ռ�������0.896L����״̬�£���
��3�������ݼ�����BaCl2�ø������6.27g����������������ϴ�Ӹ����ʣ2.33g��
�Իش��й����⣺
���ж�K+��Cl���Ƿ���ڣ�K+ Cl�� ����������ţ�
A��һ������ B�����ܴ��� C��һ��������
�ڿ϶����ڵ����ӵ�ԭ��Һ�е����ʵ���Ũ�ȷֱ�Ϊ
��
��12�֣�
��1��A B ��2��NH4+ 0.4mol/L SO42- 0.1mol/L CO32- 0.2mol/L K+ >=0.2mol/L
����

��ϰ��ϵ�д�
�����Ŀ
����һ������ˮ��Һ���������������е������֣�Na+��NH+4��Cl-��Ba2+��HCO3-��SO42-����ȡ����100mL����Һ��������ʵ�飺
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ�����ȣ��ռ�����״���µ�����448mL
��3�������ݼ�����Ba��OH��2��Һ���ó���4.30g��������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ���ǣ�������
��1����һ�ݼ���AgNO3��Һ�г�������
��2���ڶ��ݼ�����NaOH��Һ�����ȣ��ռ�����״���µ�����448mL
��3�������ݼ�����Ba��OH��2��Һ���ó���4.30g��������������ϴ�ӡ������������Ϊ2.33g����������ʵ�飬�����Ʋ���ȷ���ǣ�������
| A��Na+һ������ | B��100mL����Һ�к�0.1mol HCO-3 | C��Ba2+һ������ | D��Cl-һ�������� |
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�Na+��NH4+��Ba2+��Cl-��CO32-��SO42-����ȡ����200mL��Һ��������ʵ�飬����ʵ�������Ʋ���ȷ���ǣ�������
�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������1.36g��
�ڵڶ��ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g��
�ٵ�һ�ݼ�����NaOH��Һ�����ȣ��ռ�������1.36g��
�ڵڶ��ݼ�����BaCl2��Һ�ø������12.54g������������ϴ�ӡ������������Ϊ4.66g��
| A��һ��������Ba2+��NH4+���ܴ��� | B��CO32-һ�������� | C��Na+һ������ | D��һ��������Cl- |