��Ŀ����
ijʵ��С����̽��Na2CO3��NaHCO3�����ʣ�����ʵ������ʢ�����ֹ�����Լ�ƿ��ʧ�˱�ǩ�����ǣ������ȶԹ���A��B���м�����ͨ��ʵ���������̽����
��1���ֱ���ȹ���A��B�����ֹ���A���Ȳ�����������ʹ����ʯ��ˮ����ǡ�A���ȷֽ�Ļ�ѧ��
��ʽΪ ��
��2����ȡ���ֹ����2 g���ֱ��������С�ձ��У��ٸ���10 mL ����ˮ���������¶ȱ仯����
�������ܽ⣬�ָ������£���������Һ�и�����2�η�̪��Һ��
�ٷ���Na2CO3������ȫ�ܽ⣬��NaHCO3������ʣ�࣬�ɴ˵ó����� ��
��ͬѧ�������ձ��л��۲쵽�����������У�ʢ��Na2CO3���ձ��г��ֵ������� ������ĸ��ţ���
a����Һ�¶��½� b����Һ�¶�����
c�������̪���dz��ɫ d�������̪��ʺ�ɫ
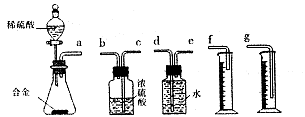
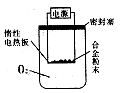
��3����ͼ��ʾ�������������õ�װ�â�͢��зֱ����ҩƷ���������ڵĹ���ͬʱ�����Թ��С�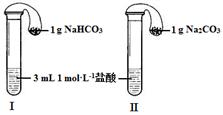
�����Թ��о��������壬 ������ķ�Ӧ�̶ȸ�Ϊ���ҡ�
�ڷ�Ӧ����������������ͣ��ָ������£�����˵����ȷ���� ��
a��װ�â����������ϴ� b��װ�â����������ϴ�
c������������������������� d�����������������ݹ������
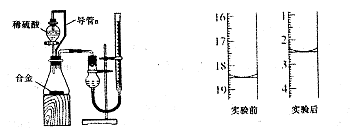
��4��ͬѧ�ǽ����ֹ���ֱ����Ƴ�0.5 mol��L-1����Һ��������·������Է�Ӧ��������Ԥ�⣺
| ʵ�鷽�� | Ԥ������ | Ԥ������ |
| ����1����2 mL Na2CO3��Һ�еμ�1 mL 0.5 mol��L-1CaCl2��Һ | �а�ɫ ���� | Na2CO3��Һ�е�CO32-Ũ�Ƚϴ�����CaCl2������Ӧ ��д���ӷ���ʽ���� |
| ����2����2 mL NaHCO3��Һ�еμ�1 mL 0.5 mol��L-1CaCl2��Һ | �ް�ɫ ���� | NaHCO3��Һ�е�CO32-Ũ�Ⱥ�С��������CaCl2��Ӧ�� |
ʵʩʵ����ֲ���2��������Ԥ���в��죺������ɫ���������塣��������£�NaHCO3��Һ��
CaCl2��Һ��Ӧ�����ӷ���ʽΪ ��
��1�� 2 NaHCO3  Na2CO3+CO2��+ H2O��2�֣�
Na2CO3+CO2��+ H2O��2�֣�
��2���� ͬ�¶��£�Na2CO3��NaHCO3������ˮ��2�֣���˵���¶������Ŀ�1�֣�
��b��d��2�֣���3���٢�1�֣� ��a��c��2�֣�
��4�� Ca2++CO32-��CaCO3����1�֣� Ca2++2HCO3-��CaCO3��+ CO2��+ H2O��2�֣�
���������������1��̼�����Ʋ��ȶ��������ֽ�����̼���ơ�CO2��ˮ����Ӧ�Ļ�ѧ����ʽΪ2 NaHCO3  Na2CO3+CO2��+ H2O��
Na2CO3+CO2��+ H2O��
��2����Na2CO3������ȫ�ܽ⣬��NaHCO3������ʣ�࣬��˵������ͬ�¶��£�̼���Ʊ�̼������������ˮ��
��̼��������ˮ�Ƿ��ȹ��̣�a����b��ȷ��̼��������ˮCO32��ˮ�⣬��Һ�����ԣ������ʹ��̪��Һ�Ժ�ɫ��c����ȷ��d��ȷ����ѡbd��
��3����̼����������ķ�Ӧ�Ƿֲ����еģ���Ӧ�ķ���ʽ�ֱ�ΪNa2CO3��HCl=NaCl��NaHCO3��NaHCO3��HCl=NaCl��H2O��CO2��������̼�����������ᷴӦ�����ң���ѡI��
����������ʵ�����0.003L��1mol/L��0.003mol����ȫ��Ӧ����̼���ƺ�̼�����Ƶ������ֱ���0.003mol��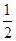 ��106g/mol��0.159g��0.003mol��84g/mol��0.252g������ڷ�Ӧ��������Dz���ģ����Լ�������������������������㣬c��ȷ��d����ȷ��������������ȵ������£�̼�����Ƶ���Է�������С��̼���Ƶģ����̼�����Ʒų���CO2��������࣬����װ�â����������ϴ�a��ȷ��b����ȷ����ѡac��
��106g/mol��0.159g��0.003mol��84g/mol��0.252g������ڷ�Ӧ��������Dz���ģ����Լ�������������������������㣬c��ȷ��d����ȷ��������������ȵ������£�̼�����Ƶ���Է�������С��̼���Ƶģ����̼�����Ʒų���CO2��������࣬����װ�â����������ϴ�a��ȷ��b����ȷ����ѡac��
��4������I�еİ�ɫ������̼��ƣ���˵��̼�����ܺ��Ȼ��Ʒ�Ӧ����̼��ư�ɫ��������Ӧ�����ӷ���ʽΪCa2++CO32-��CaCO3����������ɫ���������壬��˵����ɫ������̼��ƣ�������CO2����˸������£�NaHCO3��Һ��CaCl2��Һ��Ӧ�����ӷ���ʽΪCa2++2HCO3-��CaCO3��+ CO2��+ H2O��
���㣺����̼������̼�����Ƶ����ʱȽϡ����������ᷴӦ���йؼ����Լ�ʵ�鷽����������۵�

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
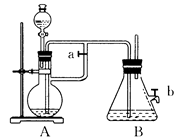
������ӱ������ͯ������ϵ�д���12�֣�ij��ȤС���о�SO2���廹ԭFe3+��I2������ʹ�õ�ҩƷ��װ������ͼ��ʾ��
��1��SO2������Fe3+��Ӧ����Ҫ������__ __ ���������ӷ��ţ�
��2������ʵ�鷽������������ʵ������ȡ����SO2���� ��
| A��Na2SO3��Һ��HNO3 | B��Na2SO3������Ũ���� |
| C���������ڴ�����ȼ�� | D��ͭ����ŨH2SO4 |
��4�������280mL SO2���壨������Ϊ��̬������Cװ���У���C��50mL NaOH��Һ�����ʵ���Ũ������Ϊ mol/L���ܴﵽĿ�ġ�
��5��������װ����ͨ�������SO2��Ϊ����֤A��SO2��Fe3+������������ԭ��Ӧ������ȡA�е���Һ���ֳ����ݣ������������ʵ�飺
�����٣�����һ����Һ�м���KMnO4��Һ���Ϻ�ɫ��ȥ��
�����ڣ����ڶ�����Һ����KSCN��Һ������죬�ټ������Ƶ���ˮ����Һ��졣
�����ۣ�����������Һ������ϡ�����ữ��BaCl2��������ɫ������
������������������ ��ԭ���� ��
��6���ܱ���I���Ļ�ԭ������SO2�������� ��д���й����ӷ���ʽ�� ��
������Ŀ�ĵ����������������е�һ�ֶԲ������п�ʴ���Ƴɣ����������ǣ�������
| A������ | B������� | C���ռ� | D������ |

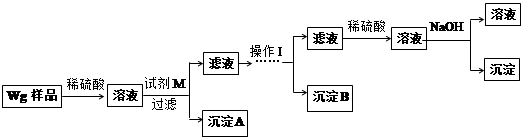
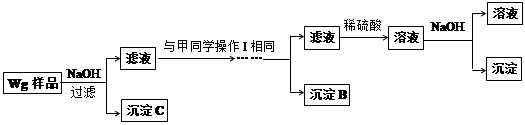

 ����ԭ��Mn2+����Ӧ�����Һ�м�������п�ۣ�����ɫ�պ���ʧ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ�
����ԭ��Mn2+����Ӧ�����Һ�м�������п�ۣ�����ɫ�պ���ʧ��������Һ�ռ�����ƿ�У���ʱ��Һ�Գ����ԡ� ��
��
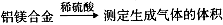 ��
��