��Ŀ����
����������۵���16.8 �棬�е�44.8 �档ʵ���Һϳ�SO3��װ����ͼ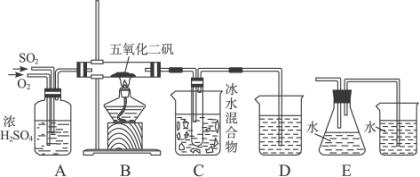
��ش���������
��1��װ��A������_______________________________________________��
��2����Ӧ����һ��ʱ���C�пɹ۲쵽��������________________________________��
��3������C��ΪE��ʵ������пɹ۲쵽��������________________________________��������Ϊ________________________________________________________��
��4��ʹSO2��O2�������1��1��ϣ��ҿ���ͨ��B������ʹ֮��ַ�Ӧ����C������������__________________��������ڵ�ԭ����_______________________________��
��5��д��B�з�����Ӧ�Ļ�ѧ����ʽΪ________________________________��
��1���ٸ������壬��ʹ���������Ͼ��ȣ���ͨ���۲����ݿ�����������
��2������ɫ��������
��3��Һ�������������� SO3��H2O���ҷ�Ӧ���ų��������ȣ��γ�����
��4��O2��SO3��SO2��SO2��O2�ķ�ӦΪ���淴Ӧ��������ȫת����
��5��2SO2+O2![]() 2SO3
2SO3

��ϰ��ϵ�д�
�����Ŀ




