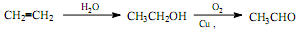��Ŀ����
��15�֣�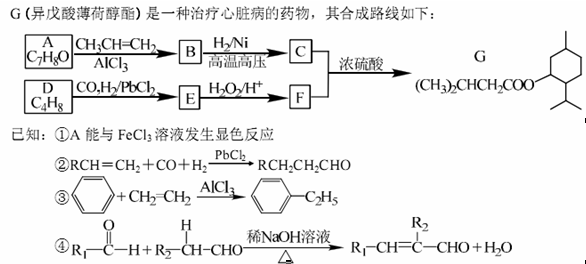
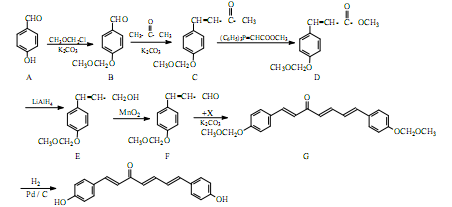
��1�� A������Ϊ�ߣߣߣ�
��2�� G�к�������������Ϊ�ߣߣߣ�
��3�� D�ķ����к��Уߣߣߣ��ֲ�ͬ��ѧ��������ԭ��
��4�� E�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽΪ�ߣߣߣ�
��5��д����������������A������ͬ���칹��Ľṹ��ʽ���ߣߣߣ�
A����������6��̼ԭ����һ��ֱ���ϣ�b�������к�����OH
��6�����촼���������ϡ��ٽ����ȣ�д�����Ҵ�Ϊԭ���Ʊ�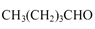 �ĺϳ�·����
�ĺϳ�·����
��ͼ�����Լ����ã����ϳ�·������ʾ��ͼʾ�����£�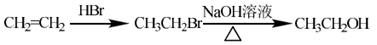
��15�֣�
��1��3�������ӣ��������ӣ���2�֣� ��2��������1�֣�
��3��2��2�֣�
��4��(CH3)2CHCH2CHO+2Cu(OH)2+NaOH (CH3)2CHCH2COONa+Cu2O��+3H2O
(CH3)2CHCH2COONa+Cu2O��+3H2O 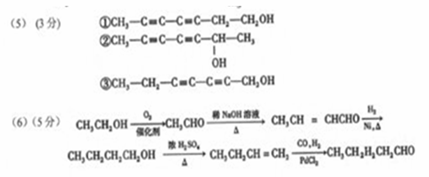
�������������A����FeCl3��Һ������ɫ��Ӧ��˵��A���з��ǻ����Ա�G�Ľṹ���ɵ�A�Ľṹ��ʽΪ�� 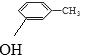 ������Ϣ�ۣ�A��CH3CH=CH2��AlCl3�����·�����������Ӧ���õ�BΪ��
������Ϣ�ۣ�A��CH3CH=CH2��AlCl3�����·�����������Ӧ���õ�BΪ��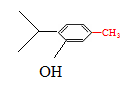 ������Ϣ�ں�G�Ľṹ��֪DΪ��(CH3)2C=CH2��D��CO��H2��PbCl2���������ɵ�EΪ��(CH3)2CHCH2CHO��E��H2O2����Ϊ���ᣬFΪ��(CH3)2CH CH2COOH��
������Ϣ�ں�G�Ľṹ��֪DΪ��(CH3)2C=CH2��D��CO��H2��PbCl2���������ɵ�EΪ��(CH3)2CHCH2CHO��E��H2O2����Ϊ���ᣬFΪ��(CH3)2CH CH2COOH��
��1��A�Ľṹ��ʽΪ��  ������Ϊ��3�������ӣ��������ӣ���
������Ϊ��3�������ӣ��������ӣ���
��2������G�Ľṹ��ʽ��֪G�к�������������Ϊ������
��3��D�Ľṹ��ʽΪ��(CH3)2C=CH2������D�ķ����к���2�ֲ�ͬ��ѧ��������ԭ�ӡ�
��4��EΪ��(CH3)2CHCH2CHO������ȩ�����������Ƶ�������ͭ��������ѧ����ʽΪ��
(CH3)2CHCH2CHO+2Cu(OH)2+NaOH (CH3)2CHCH2COONa+Cu2O��+3H2O
(CH3)2CHCH2COONa+Cu2O��+3H2O
��5������a����������6��̼ԭ����һ��ֱ���ϣ�b�������к�����OH����֪a���Ӻ�������̼̼���������������ֽṹ����CH3��C��C��C��C��CH2��CH2OH����CH3��C��C��C��C��C(OH)��CH3����CH3��CH2��C��C��C��C��CH2OH
��6��CH3CH2OH�ڴ�����������O2��Ӧ����CH3CHO��������Ϣ�ܣ�CH3CHO������ȩ����ת��Ϊ��CH3CH=CHCHO����H2�ӳ�ת��ΪCH3CH2CH2CH2OH��������ȥ��Ӧ����CH3CH2CH=CH2��������Ϣ�ڣ���CO��H2��PbCl2����������CH3CH2CH2CH2CHO�����Ժϳ�·������ʾ��ͼΪ��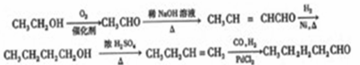
���㣺���⿼���л��ϳɵķ������ƶϡ�ͬ���칹�塢��ѧ����ʽ����д��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�[15��]������X��һ�ֻ������أ���������ת����ϵ��
������A����FeCl3��Һ������ɫ��Ӧ�������к���������ѧ������ȫ��ͬ�ļ����䱽���ϵ�һ����ȡ����ֻ�����֡�1H-NMR����ʾ������G��������ԭ�ӻ�ѧ������ͬ��F��һ�ֿ������Ʊ������۾��ĸ߾��
����������Ϣ�ش��������⡣
��1������������ȷ����_________��
| A��������A�����к��������ṹ��Ԫ |
| B��������A���Ժ�NaHCO3��Һ��Ӧ���ų�CO2���� |
| C��X��NaOH��Һ��Ӧ��������1 mol X�������6 mol NaOH |
| D��������D����Br2�����ӳɷ�Ӧ |
��3��д��ͬʱ��������������D������ͬ���칹��Ľṹ��ʽ�������������칹_________��
a���������� b���ܷ���������Ӧ
��4��д��B��C��Ӧ�Ļ�ѧ����ʽ_______________________________________________��
��5��д��E��F��Ӧ�Ļ�ѧ����ʽ_______________________________________________��
����ʵ���У���ѡװ�ò���������

| A�������ᴿ��ѡ�ٺ͢� |
| B����CCl4��ȡ��ˮ�еĵ⣬ѡ�� |
| C������Na2CO3��Һ��CH3COOC2H5��ѡ�� |
| D����NaOH��Һ����Cl2��ѡ�� |
���б仯������з�����ѧ�仯����
| A�����Ĺ̶� | B�������ЧӦ |
| C��ʵ��������ȡ����ˮ | D���ò�˿պȡNaCl��Һ������ɫ��Ӧ |
�����й�ʵ���˵������ȷ����
| A��ֽ����������Cu2+��Fe3+���Һ����ʱ��Fe3+�ڹ̶����з�������ϵ� |
| B���õ�����ƽ������ѧҩƷʱ�������ȳ�С�ձ����������ٳ��������Լ����������������֮�ΪҩƷ������ |
| C�����������3mol��L-1������Һ��Ӧ���Թ��м�������NaCl���壬��Ӧ���ʻ�ӿ� |
| D�������������Ȼ�������ʱ�������������ƾ������ѵĻ�������ⶾ |


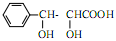 �Ǻϳ���ɼ���Ĺؼ��������д����
�Ǻϳ���ɼ���Ĺؼ��������д����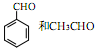 Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�
Ϊԭ���Ʊ��û�����ĺϳ�·������ͼ�����Լ����ã����ϳ�·������ͼʾ�����£�