��Ŀ����
��ҵ������ʱ�����ô�������Ӧ��SO2ת��ΪSO3��һ���ؼ����衣
(1)ij�¶��£�SO2(g)+1/2O2(g) SO3(g)��H=-98 kJ/mol����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�196kJ�����������¶��µ�ƽ�ⳣ��K=_________��
SO3(g)��H=-98 kJ/mol����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�196kJ�����������¶��µ�ƽ�ⳣ��K=_________��
(2)һ�������£���һ���������ܱ������г���2 mol SO2��1 mol O2���������з�Ӧ��2SO2(g)+O2(g)
 2SO3(g)���ﵽƽ��ı�����������SO2��O2��SO3��ƽ��Ũ�ȶ���ԭ���������_______������ĸ����
2SO3(g)���ﵽƽ��ı�����������SO2��O2��SO3��ƽ��Ũ�ȶ���ԭ���������_______������ĸ����
A�������¶Ⱥ�����������䣬����2 mol SO3
B�������¶Ⱥ�����������䣬����2 mol N2
C�������¶Ⱥ�����������䣬����0.5 mol SO2��0.25 mol O2
D�������¶Ⱥ�������ѹǿ���䣬����1 mol SO3
E�������¶�
F���ƶ�����ѹ������
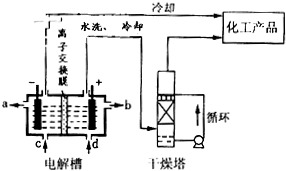
(3)ij����������ͼ��ʾװ���õ绯ѧԭ���������ᣬд��ͨ��SO2�ĵ缫�ĵ缫��Ӧʽ��____________
(1)ij�¶��£�SO2(g)+1/2O2(g)
 SO3(g)��H=-98 kJ/mol����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�196kJ�����������¶��µ�ƽ�ⳣ��K=_________��
SO3(g)��H=-98 kJ/mol����ʼʱ��100 L���ܱ������м���4.0 mol SO2(g)��10.0 mol O2(g)������Ӧ�ﵽƽ��ʱ���ų�196kJ�����������¶��µ�ƽ�ⳣ��K=_________�� (2)һ�������£���һ���������ܱ������г���2 mol SO2��1 mol O2���������з�Ӧ��2SO2(g)+O2(g)
 2SO3(g)���ﵽƽ��ı�����������SO2��O2��SO3��ƽ��Ũ�ȶ���ԭ���������_______������ĸ����
2SO3(g)���ﵽƽ��ı�����������SO2��O2��SO3��ƽ��Ũ�ȶ���ԭ���������_______������ĸ���� A�������¶Ⱥ�����������䣬����2 mol SO3
B�������¶Ⱥ�����������䣬����2 mol N2
C�������¶Ⱥ�����������䣬����0.5 mol SO2��0.25 mol O2
D�������¶Ⱥ�������ѹǿ���䣬����1 mol SO3
E�������¶�
F���ƶ�����ѹ������
(3)ij����������ͼ��ʾװ���õ绯ѧԭ���������ᣬд��ͨ��SO2�ĵ缫�ĵ缫��Ӧʽ��____________

(4)����ʱ��BaSO4��Ksp=1.08��10-10���ֽ��������BaCl2��Һ��2.0��10-3mol/L��Na2SO4��Һ��ϡ���Ҫ ����BaSO4������BaCl2��Һ����СŨ��Ϊ___________��
(5)���й���2SO2(g)+O2(g) 2SO3(g)��Ӧ��ͼ���У���ȷ����______________��
2SO3(g)��Ӧ��ͼ���У���ȷ����______________��
(5)�����2SO2(g)+O2(g)
 2SO3(g)��Ӧ��ͼ���У���ȷ����______________��
2SO3(g)��Ӧ��ͼ���У���ȷ����______________�� 
(6)SO2�������������л�ԭ�ԣ�����Ư���ԡ���SO2ͨ������KMnO4��Һ�У�����KMnO4��Һ��ɫ��SO2����______�ԣ������������ͻ�ԭ�������ʵ���֮��Ϊ_______����֪��KMnO4�Ļ�ԭ����ΪMn2+����
(1)10/3��3.33
(2)ACF
(3)SO2+2H2O-2e-=SO42-+4H+
(4)2. 16��10-7mol/L
(5)ABD
(6)��ԭ��2��5
(2)ACF
(3)SO2+2H2O-2e-=SO42-+4H+
(4)2. 16��10-7mol/L
(5)ABD
(6)��ԭ��2��5

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ