��Ŀ����
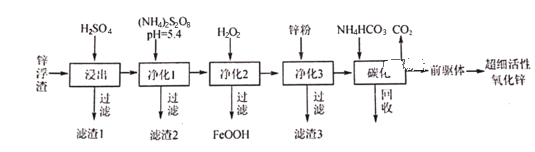
����Ŀ��п������Ҫ��Zn��ZnO��SiO2��Fe2+��Cd2+��Mn2+����ҵ�Ͽ�ͨ������������һ��ȥ�������Ʊ���ϸ��������п���乤���������£�
(1)����1�ijɷ�Ϊ___________��
(2)��S2O82���Ľṹʽ��ֻ����һ����OһO���Ǽ��Լ�����S�Ļ��ϼ�Ϊ___________����ҵ�ϳ��ö��Ե缫���(NH4)2SO4����(NH4)2S2O8(���������)�������缫��ӦʽΪ__________________������1��Ϊ�˽�Mn2+ת��ΪMnO2����ȥ��д���÷�Ӧ�����ӷ���ʽ��______________________��
(3)����3��Ŀ��_________________________________��
(4)̼��������Һ����Ҫ�ɷ�Ϊ___________��������ѭ��ʹ�õ�Ŀ��___________________��
(5)̼����50����У���ǰ���塱�Ļ�ѧʽΪZnCO3��2Zn(OH)2��H2O��д��̼���������ɡ�ǰ���塱�Ļ�ѧ����ʽ��__________________________________________________��
���𰸡���������(��SiO2) +6 2SO42- -2e-=S2O82- Mn2��+S2O82-+2H2O=MnO2��+2SO42- +4H�� ��ȥ��Һ�е�Cd2+ (NH4)2SO4 �������Ʊ�(NH4)2S2O8(���������) 3ZnSO4+6NH4HCO3=ZnCO3��2Zn(OH)2��H2O��+3(NH4)2SO4+5CO2��
��������
������п���������������ﲻ�ܷ�Ӧ��������������P�Ƚϻ��õĽ�����Ӧ��SiO2���ܷ�Ӧ��������1��ʽ���ڣ���п��ˮ����Ҫ�� Zn2+��Fe2+��Cd2+��Mn2+������(NH4)2S2O8�� Mn2+ת��ΪMnO2��ȥ������2Ϊ�������̣���2��Һ�м���H2O2�����Խ�+2�۵�Fe����Ϊ+3�۵�Fe���γ�FeOOH����������3����Һ�м���п��ԭCd2+���ɵ��ʸ������˵õ�����3Ϊ����Ȼ������Һ�м���̼�����̼���� 50����еõ�[ZnCO32Zn��OH��2H2O]��̼��ʱ���� NH4HCO3 ��ʵ������Ϊ���������� 1.1 ������������ʹ Zn2+��ֳ������ҽϸ��¶���̼����立ֽ����ʧ���ݴ˷�����
(1)п������Ҫ��Zn��ZnO��SiO2�����ʣ�����ϡ���ᣬZn��ZnO�����ᷴӦ��ΪZn2+������Һ����SiO2��������������������ᷴӦ����������1��Ҫ�ɷ��Ƕ������裻
(2)��S2O82-�Ľṹʽ��ֻ����һ����OһO���Ǽ��Լ�,��S��Sԭ��֮�����һ����O��O����Sԭ�����ĸ�Oԭ���γɹ��ۼ���ÿ��Sԭ���γ�����S=O˫��������S-O�����������������ӵ�����O>S������SԪ�صĻ��ϼ�Ϊ+6�ۣ��ö��Ե缫���(NH4)2SO4����(NH4)2S2O8(���������)��SO42-��������ʧȥ���ӣ���ΪS2O82-�������缫��ӦʽΪ��2SO42- -2e-=S2O82-������1����Һ�к���Zn2+��Mn2+��Fe2+��Cd2+����1��Һ�м���(NH4)2S2O8��Һ��(NH4)2S2O8��Mn2+����ת��ΪMnO2�γ�����2�����˳�ȥ��S2O82-���õ��ӱ�ΪSO42-������Һ���÷�Ӧ�����ӷ���ʽΪMn2��+S2O82-+2H2O=MnO2��+2SO42- +4H����
(3)����2��Ŀ���Ǽ���H2O2����Һ�е�Fe2+����Ϊ+3�۵���������Һ�е�OH-����γ�FeOOH������ȥ����3��Һ�м���Zn�ۣ�Zn+Cd2+=Zn2++Cd��Ȼ����ˣ����Խ�������Zn���û�����Cd���ʹ��˳�ȥ����������3�ɷ�ΪZn��Cd��
(4)����3�Ժ����Һ�к���Zn2+�������Һ�м���̼�����̼�����õ�����狀Ͷ�����̼����ǰ���������仯ѧʽΪZnCO32Zn(OH)2H2O����Ӧ����ʽ��3ZnSO4+6NH4HCO3=ZnCO32Zn(OH)2H2O��+3(NH4)2SO4+5CO2�����γɳ�������ˣ��õ�����Һ����Ҫ������(NH4)2SO4�������ʺ���NԪ�أ���ֱ���������ʣ�Ҳ��������ѭ��ʹ���Ʊ�(NH4)2S2O8(���������)��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�



