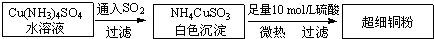��Ŀ����
�Ȼ���ͭ��CuCl������Ҫ�Ļ���ԭ�ϣ����ұ��涨�ϸ��CuCl��Ʒ����Ҫ����ָ��ΪCuCl��������������96.5%����ҵ�ϳ�ͨ�����з�Ӧ�Ʊ�CuCl��2CuSO4+Na2SO3+2NaCl+Na2CO3�T2CuCl��+3Na2SO4+CO2��
��1��CuCl�Ʊ���������Ҫ������������Ϊ20.0%��CuSO4��Һ���Լ������Ƹ���Һ�����CuSO4?5H2O��H2O������֮�ȣ�
��2��ȷ��ȡ���Ʊ���0.250 0g CuCl��Ʒ����һ������0.5 mol?L-1 FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000 mol?L-1 Ce��SO4��2��Һ�ζ����յ㣬����24.60mL Ce��SO4��2��Һ���йط�Ӧ�����ӷ���ʽΪ��Fe3++CuCl�TFe2++Cu2++Cl-��Ce4++Fe2+�TFe3++Ce3+ͨ������˵��������Ʒ��CuCl�����������Ƿ���ϱ���
��1��CuCl�Ʊ���������Ҫ������������Ϊ20.0%��CuSO4��Һ���Լ������Ƹ���Һ�����CuSO4?5H2O��H2O������֮�ȣ�
��2��ȷ��ȡ���Ʊ���0.250 0g CuCl��Ʒ����һ������0.5 mol?L-1 FeCl3��Һ�У�����Ʒ��ȫ�ܽ��ˮ20mL����0.1000 mol?L-1 Ce��SO4��2��Һ�ζ����յ㣬����24.60mL Ce��SO4��2��Һ���йط�Ӧ�����ӷ���ʽΪ��Fe3++CuCl�TFe2++Cu2++Cl-��Ce4++Fe2+�TFe3++Ce3+ͨ������˵��������Ʒ��CuCl�����������Ƿ���ϱ���
��������1������ҪCuSO4?5H2O������Ϊx��H2O������Ϊy�����ݻ�ѧʽ���������ͭ������m��CuSO4������Һ����Ϊx+y
���������������з��̣�����x��y�ı�����ϵ��
��2�����ݹ�ϵʽ����n��CuCl��������������Ʒ��m��CuCl��������0.2500g�ϸ��CuCl�к���CuCl�����������бȽ��жϣ�
���������������з��̣�����x��y�ı�����ϵ��
��2�����ݹ�ϵʽ����n��CuCl��������������Ʒ��m��CuCl��������0.2500g�ϸ��CuCl�к���CuCl�����������бȽ��жϣ�
����⣺��1������ҪCuSO4?5H2O������Ϊx��H2O������Ϊy����
=20.0%
��16x=5��x+y�������� x��y=5��11
������CuSO4?5H2O��H2O������֮��Ϊ5��11��
��2������Ʒ��CuCl������Ϊx����
�ɻ�ѧ��Ӧ����ʽ��֪��CuCl������Fe2+������Ce4+
1 1
n��CuCl�� 24.60��10-3L��0.1000 mol/L
���� n��CuCl��=24.60��10-3L��0.1000 mol/L=2.46��10-3mol��
���Ը���ƷCuCl������Ϊ2.46��10-3mol��99.5g/mol=0.2448g��
0.2500g�ϸ��CuCl�к���CuCl������0.2500g��96.5%=0.2413g��С��0.2448g���ʸ���Ʒ��CuCl�������������ϱ���
����Ʒ��CuCl�������������ϱ���
| ||
| x+y |
��16x=5��x+y�������� x��y=5��11
������CuSO4?5H2O��H2O������֮��Ϊ5��11��
��2������Ʒ��CuCl������Ϊx����
�ɻ�ѧ��Ӧ����ʽ��֪��CuCl������Fe2+������Ce4+
1 1
n��CuCl�� 24.60��10-3L��0.1000 mol/L
���� n��CuCl��=24.60��10-3L��0.1000 mol/L=2.46��10-3mol��
���Ը���ƷCuCl������Ϊ2.46��10-3mol��99.5g/mol=0.2448g��
0.2500g�ϸ��CuCl�к���CuCl������0.2500g��96.5%=0.2413g��С��0.2448g���ʸ���Ʒ��CuCl�������������ϱ���
����Ʒ��CuCl�������������ϱ���
�������������ʵ���Ũ�ȼ��㡢�ζ�����ȣ��Ѷ��еȣ�ע���ڵζ������У������漰��Ӧ�϶࣬ͨ�����ù�ϵʽ���㣮

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
ijУ��ѧС���ͬѧ������̼��Ϊ�缫����Ȼ�ͭ��Һʱ��������̼���ϳ����к�ɫ���������⣬����������ɫ����������Ϊ̽������̼���ϵIJ����������¹��̣�
�������
ͭ�Ļ�������ɫ�������£�
��̽��ʵ��
��1��������裺
�ٺ�ɫ����һ����ͭ����������Cu2O��
�ڰ�ɫ����Ϊͭ�Ļ�����仯ѧʽ����ΪCuCl��
��2��ʵ����֤��
ȡ���CuCl2��Һ�������̼����ϴ�ӡ������������װ�ý���ʵ�飬��֤��������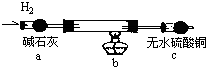
ʵ�������̼���ϵİ�ɫ���ʱ�Ϊ��ɫ����ˮ����ͭ����ɫ��
��̼���ϵĺ�ɫ�����Ƿ���Cu2O ����ǡ����������� ��
����cװ�ú�����ͨ�� �У������ְ�ɫ��������˵�����������еİ�ɫ����һ�����ڣ�
��д��װ��b�з�����Ӧ�Ļ�ѧ����ʽ�� ��
��3���������ۣ�
�ٵ��CuCl2��Һ��������Ϸ����ķ�ӦΪ��Cu2++2e-=Cu�� ��
����ʯī�缫��ⱥ������ͭ��Һ���۲�����̼��������ֵ������ɫ���ʸ��ţ��ް�ɫ���ʣ����� �������⣬���ְ�ɫ���ʣ�
�������
ͭ�Ļ�������ɫ�������£�
| ���� | ��ɫ������ | ���� | ��ɫ������ |
| ������ͭCu��OH��2 | ��ɫ���岻����ˮ | ����ͭ��CuSO4�� | ��Һ����ɫ |
| ������ͭ��CuO�� | ��ɫ���岻����ˮ | �Ȼ�ͭ��CuCl2�� | ��Һ����ɫ��ϡ��Һ����ɫ |
| �Ȼ���ͭ��CuCl�� | ��ɫ���岻����ˮ | ��ʽ�Ȼ�ͭ | ��ɫ���岻����ˮ |
��1��������裺
�ٺ�ɫ����һ����ͭ����������Cu2O��
�ڰ�ɫ����Ϊͭ�Ļ�����仯ѧʽ����ΪCuCl��
��2��ʵ����֤��
ȡ���CuCl2��Һ�������̼����ϴ�ӡ������������װ�ý���ʵ�飬��֤��������

ʵ�������̼���ϵİ�ɫ���ʱ�Ϊ��ɫ����ˮ����ͭ����ɫ��
��̼���ϵĺ�ɫ�����Ƿ���Cu2O
����cװ�ú�����ͨ��
��д��װ��b�з�����Ӧ�Ļ�ѧ����ʽ��
��3���������ۣ�
�ٵ��CuCl2��Һ��������Ϸ����ķ�ӦΪ��Cu2++2e-=Cu��
����ʯī�缫��ⱥ������ͭ��Һ���۲�����̼��������ֵ������ɫ���ʸ��ţ��ް�ɫ���ʣ�����
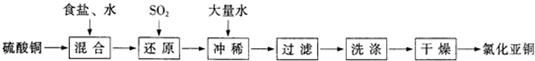
 ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�